Chemical Bonding sharing or chemical bond involves the
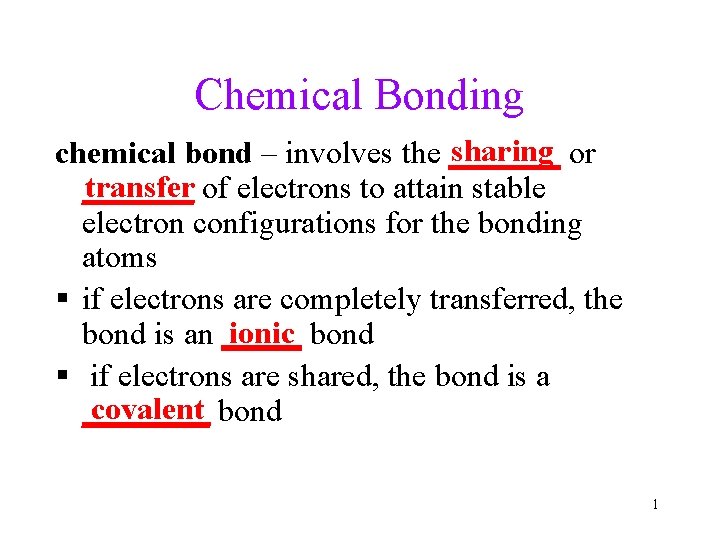
Chemical Bonding sharing or chemical bond – involves the _______ transfer of electrons to attain stable _______ electron configurations for the bonding atoms if electrons are completely transferred, the ionic bond is an _____ if electrons are shared, the bond is a covalent bond ____ 1
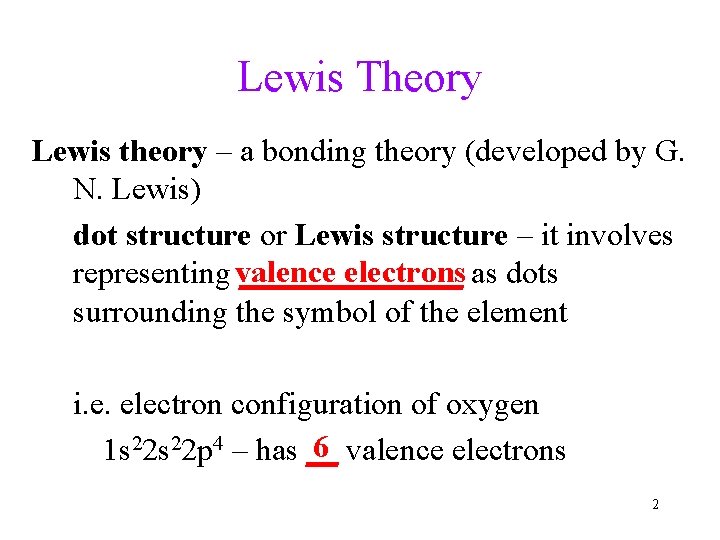
Lewis Theory Lewis theory – a bonding theory (developed by G. N. Lewis) dot structure or Lewis structure – it involves electrons as dots representing valence _______ surrounding the symbol of the element i. e. electron configuration of oxygen 6 valence electrons 1 s 22 p 4 – has __ 2

Lewis Symbols of Atoms Lewis structure: • where each dot represents a valence e– 2 dots per side • with a maximum of __ • dots are usually filled singly first, then in a pair Li • Be • • • B • • C • • • N • • • O: • • • : F: • • • : Ne: • • 3
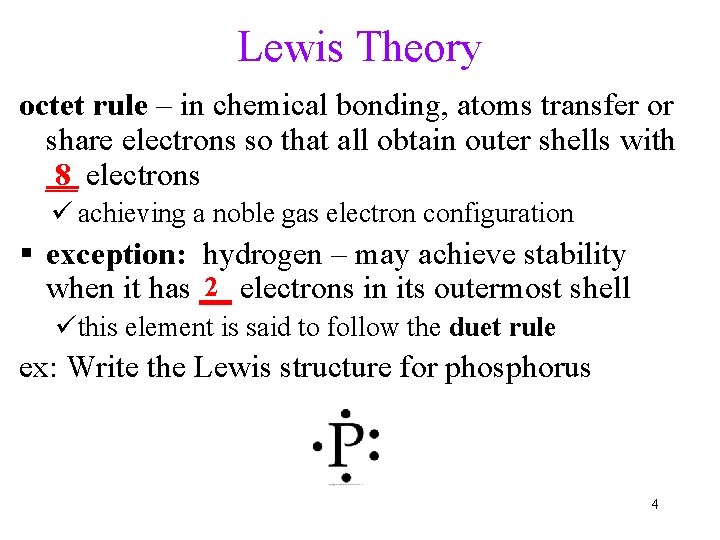
Lewis Theory octet rule – in chemical bonding, atoms transfer or share electrons so that all obtain outer shells with 8 electrons __ ü achieving a noble gas electron configuration exception: hydrogen – may achieve stability 2 electrons in its outermost shell when it has __ üthis element is said to follow the duet rule ex: Write the Lewis structure for phosphorus 4
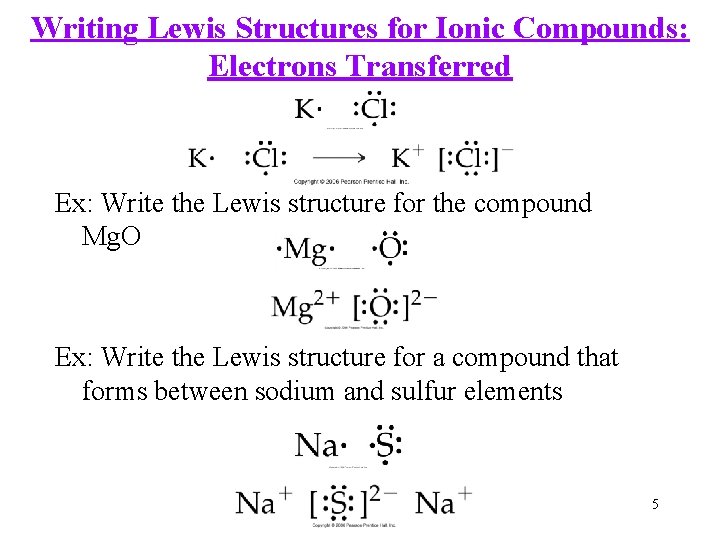
Writing Lewis Structures for Ionic Compounds: Electrons Transferred Ex: Write the Lewis structure for the compound Mg. O Ex: Write the Lewis structure for a compound that forms between sodium and sulfur elements 5
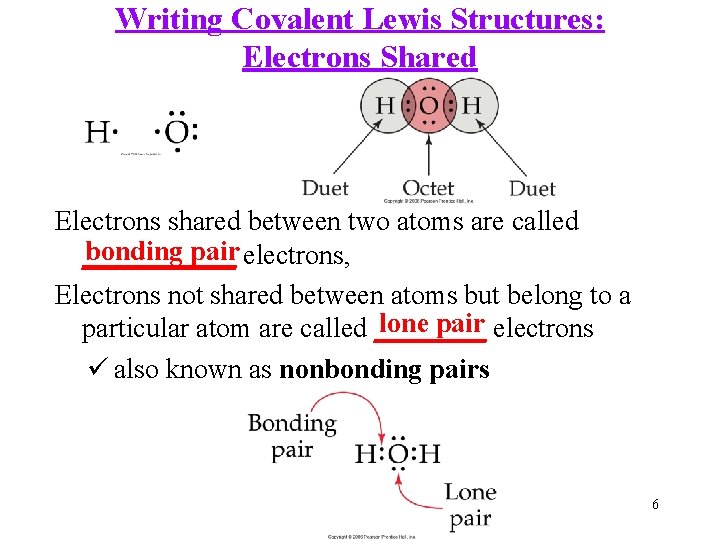
Writing Covalent Lewis Structures: Electrons Shared Electrons shared between two atoms are called bonding pair electrons, ______ Electrons not shared between atoms but belong to a lone pair electrons particular atom are called ____ ü also known as nonbonding pairs 6
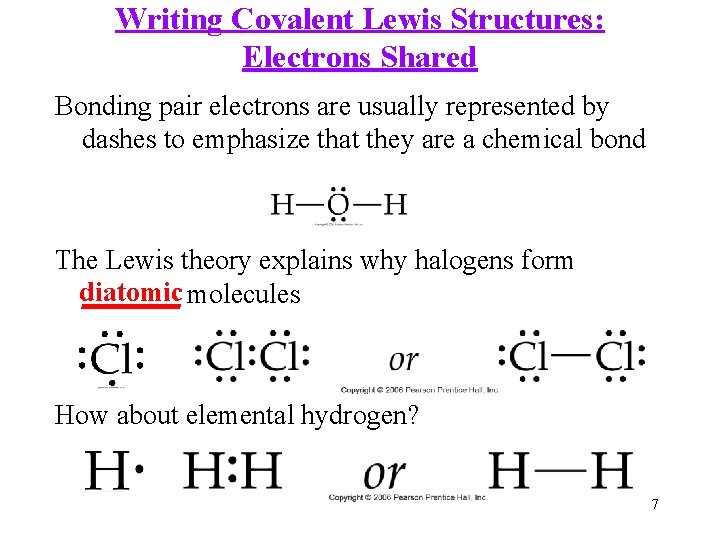
Writing Covalent Lewis Structures: Electrons Shared Bonding pair electrons are usually represented by dashes to emphasize that they are a chemical bond The Lewis theory explains why halogens form diatomic _______ molecules How about elemental hydrogen? 7

Covalent Lewis Structures: Multiple Bonds When ____ two electron pairs are shared between two double bond atoms, the resulting bond is a ______ shorter and ____ stronger than these bonds are _______ single bonds Consider oxygen gas: 8
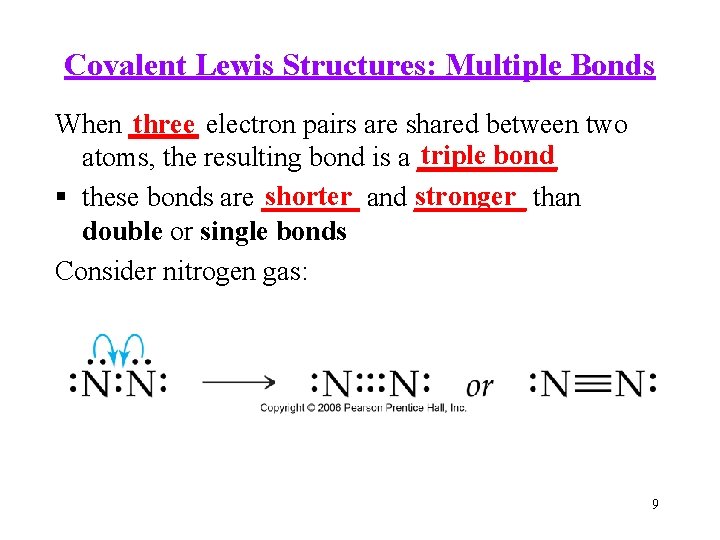
Covalent Lewis Structures: Multiple Bonds When _____ three electron pairs are shared between two triple bond atoms, the resulting bond is a _____ shorter and ____ stronger than these bonds are _______ double or single bonds Consider nitrogen gas: 9

Writing Lewis Structures for Covalent Compounds 1. Write the correct skeletal structure for the molecule most metallic element generally central many molecules tend to be symmetrical halogens and hydrogen are generally terminal (attached to the ends) in oxyacids, the acid hydrogens are attached to an oxygen Write a Lewis structure for CO 2: O C O 10
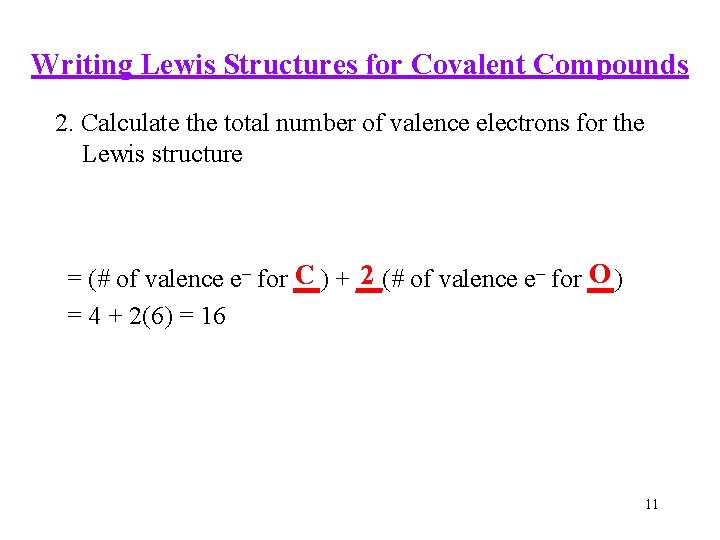
Writing Lewis Structures for Covalent Compounds 2. Calculate the total number of valence electrons for the Lewis structure O C + __(# 2 of valence e– for __) = (# of valence e– for __) = 4 + 2(6) = 16 11
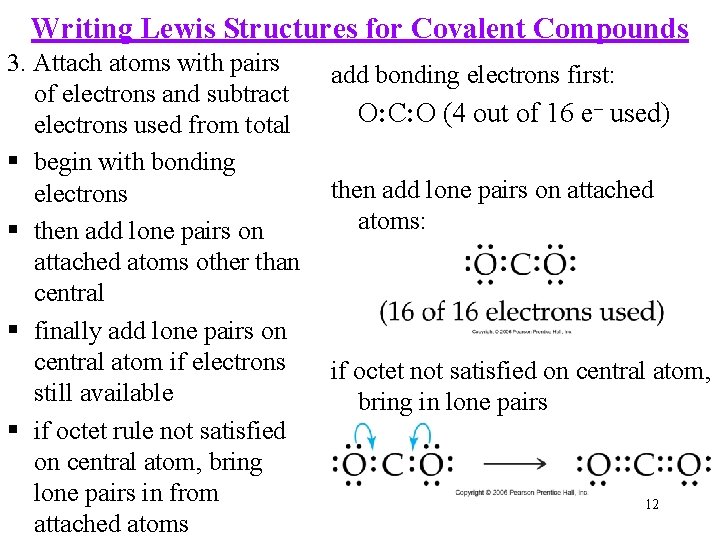
Writing Lewis Structures for Covalent Compounds 3. Attach atoms with pairs of electrons and subtract electrons used from total begin with bonding electrons then add lone pairs on attached atoms other than central finally add lone pairs on central atom if electrons still available if octet rule not satisfied on central atom, bring lone pairs in from attached atoms add bonding electrons first: O: C: O (4 out of 16 e– used) then add lone pairs on attached atoms: if octet not satisfied on central atom, bring in lone pairs 12
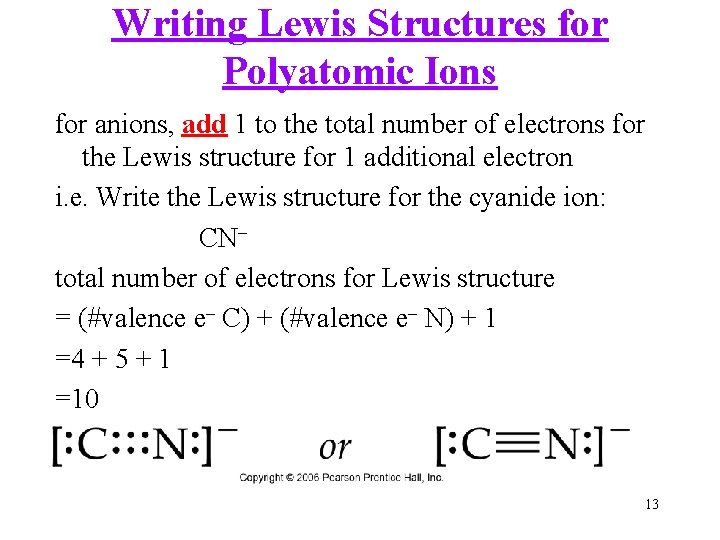
Writing Lewis Structures for Polyatomic Ions for anions, add 1 to the total number of electrons for the Lewis structure for 1 additional electron i. e. Write the Lewis structure for the cyanide ion: CN– total number of electrons for Lewis structure = (#valence e– C) + (#valence e– N) + 1 =4 + 5 + 1 =10 13
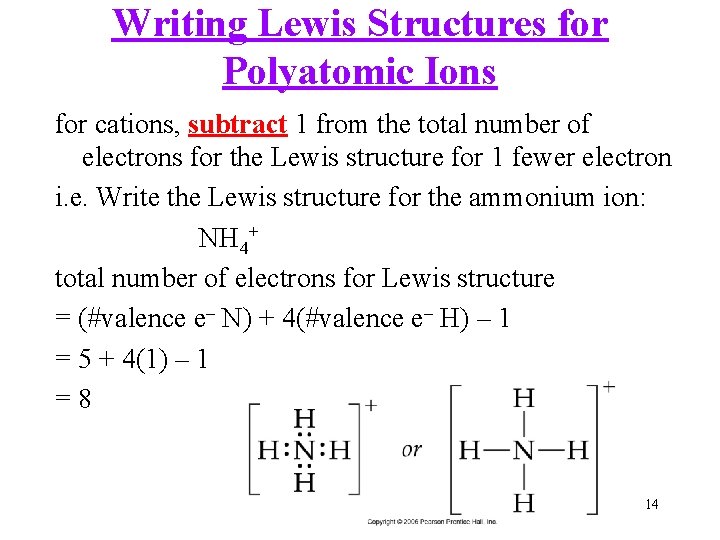
Writing Lewis Structures for Polyatomic Ions for cations, subtract 1 from the total number of electrons for the Lewis structure for 1 fewer electron i. e. Write the Lewis structure for the ammonium ion: NH 4+ total number of electrons for Lewis structure = (#valence e– N) + 4(#valence e– H) – 1 = 5 + 4(1) – 1 =8 14
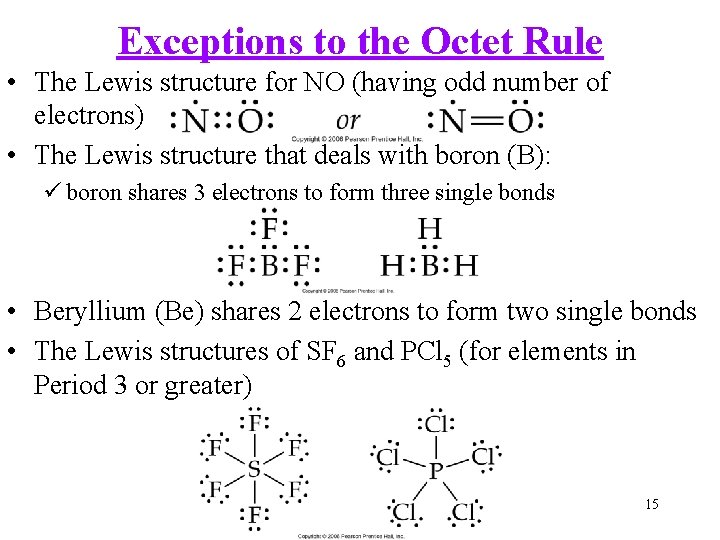
Exceptions to the Octet Rule • The Lewis structure for NO (having odd number of electrons) • The Lewis structure that deals with boron (B): ü boron shares 3 electrons to form three single bonds • Beryllium (Be) shares 2 electrons to form two single bonds • The Lewis structures of SF 6 and PCl 5 (for elements in Period 3 or greater) 15
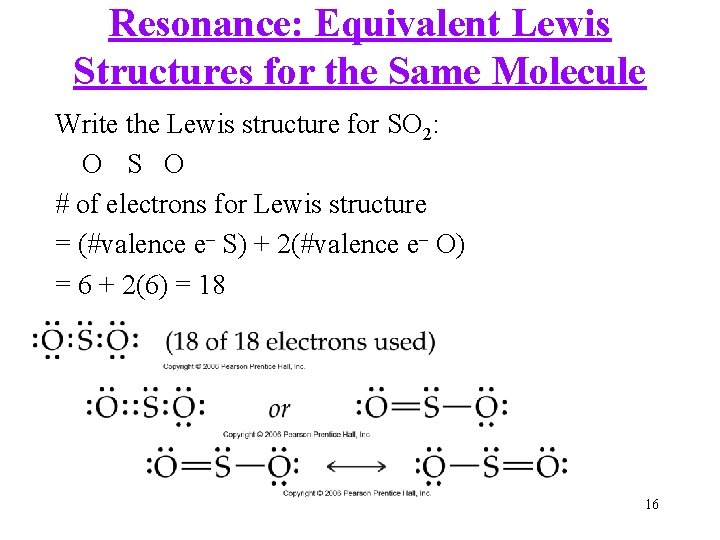
Resonance: Equivalent Lewis Structures for the Same Molecule Write the Lewis structure for SO 2: O S O # of electrons for Lewis structure = (#valence e– S) + 2(#valence e– O) = 6 + 2(6) = 18 16
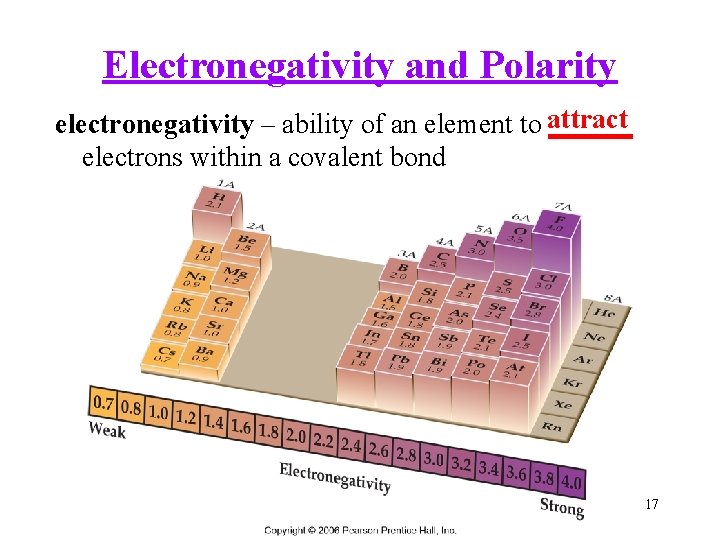
Electronegativity and Polarity electronegativity – ability of an element to attract ______ electrons within a covalent bond 17
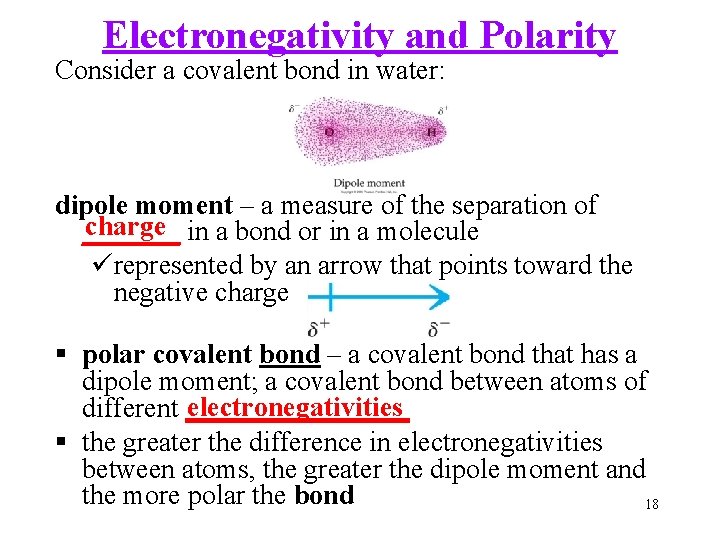
Electronegativity and Polarity Consider a covalent bond in water: dipole moment – a measure of the separation of charge in a bond or in a molecule _______ ürepresented by an arrow that points toward the negative charge polar covalent bond – a covalent bond that has a dipole moment; a covalent bond between atoms of electronegativities different ________ the greater the difference in electronegativities between atoms, the greater the dipole moment and the more polar the bond 18
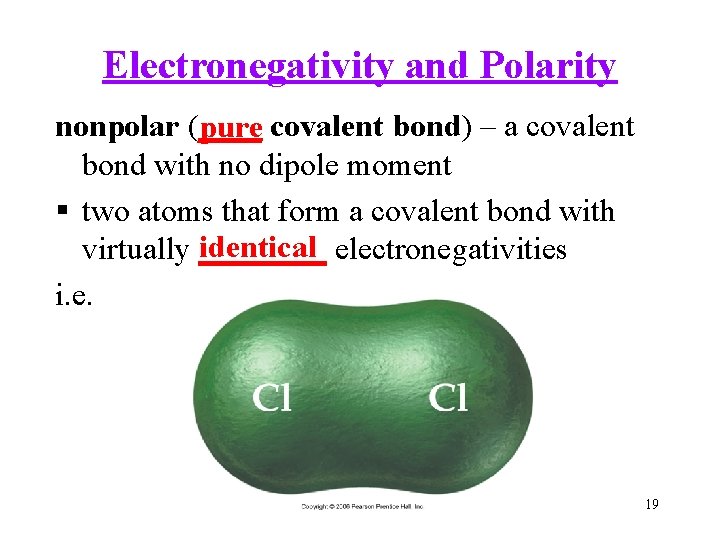
Electronegativity and Polarity nonpolar (____ pure covalent bond) – a covalent bond with no dipole moment two atoms that form a covalent bond with identical electronegativities virtually ____ i. e. 19
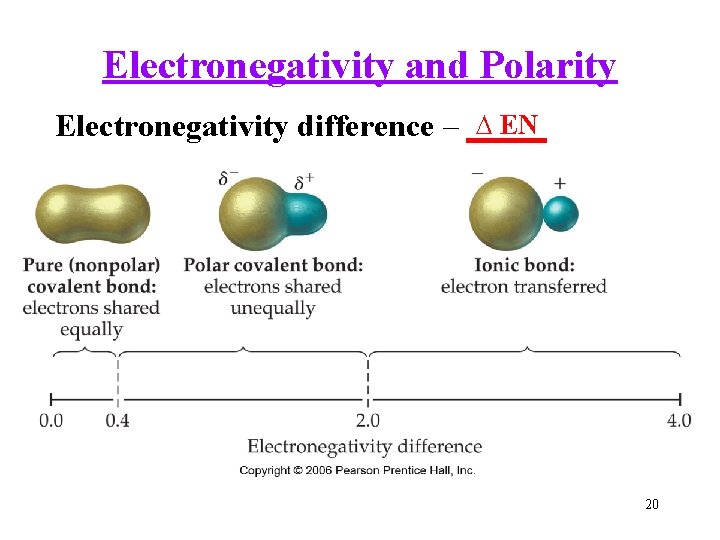
Electronegativity and Polarity ∆ EN Electronegativity difference – _____ 20
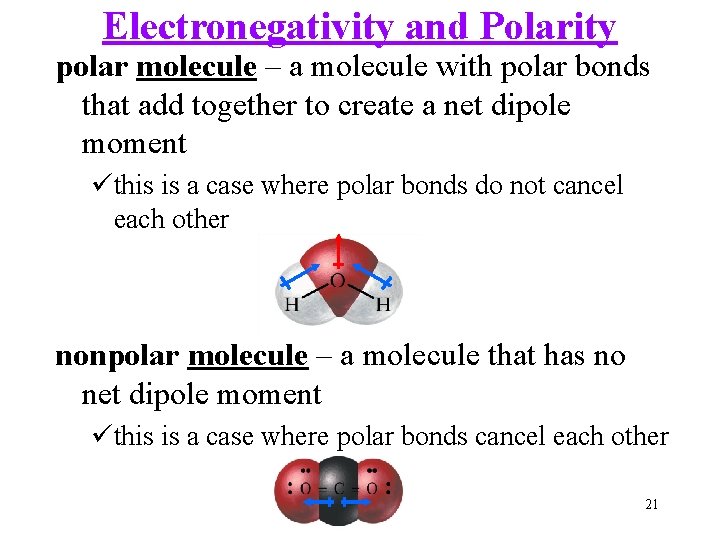
Electronegativity and Polarity polar molecule – a molecule with polar bonds that add together to create a net dipole moment üthis is a case where polar bonds do not cancel each other nonpolar molecule – a molecule that has no net dipole moment üthis is a case where polar bonds cancel each other 21
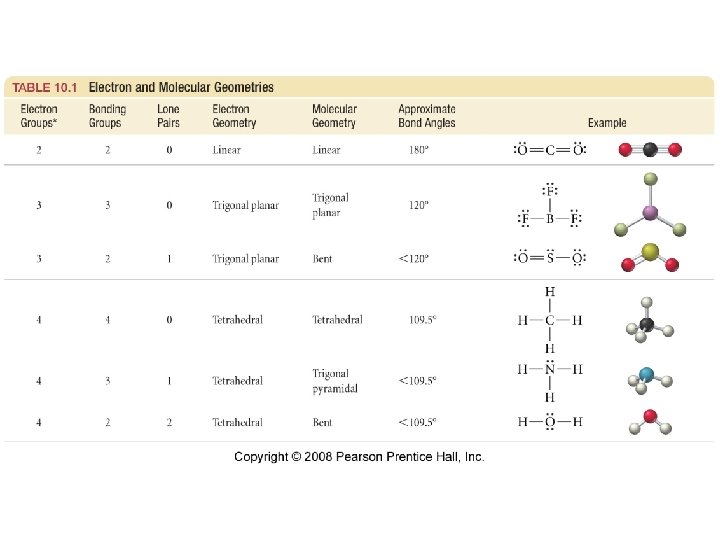
Predicting Molecular Geometry • VSEPR Theory üValence Shell Electron Pair Repulsion • The shape around the central atom(s) can be predicted by assuming that the areas of electrons on the central atom will try to get as far from each other as possible üareas of negative charge will repel each other 22
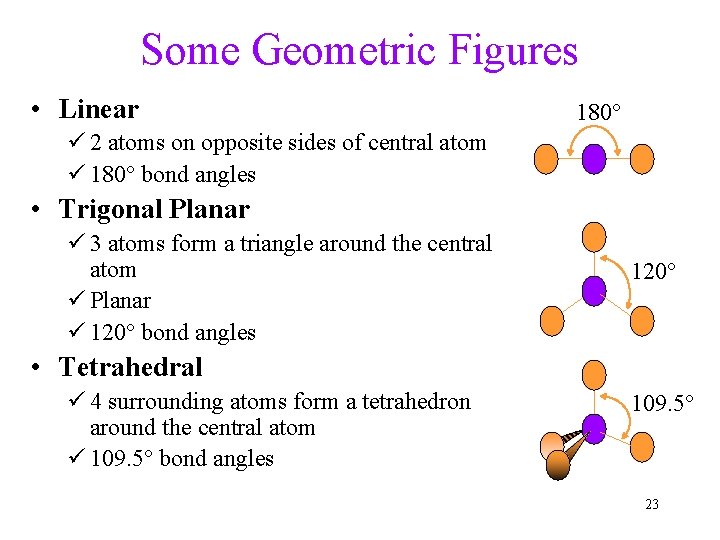
Some Geometric Figures • Linear 180° ü 2 atoms on opposite sides of central atom ü 180° bond angles • Trigonal Planar ü 3 atoms form a triangle around the central atom ü Planar ü 120° bond angles 120° • Tetrahedral ü 4 surrounding atoms form a tetrahedron around the central atom ü 109. 5° bond angles 109. 5° 23
- Slides: 24