Chemical Bonding Physical Science Metal vs Nonmetals Metals
Chemical Bonding Physical Science

Metal vs. Nonmetals • Metals – Left side of the Periodic Table • Nonmetals – Right side of the Periodic Table plus hydrogen
Valence Electrons • The number of electrons on the last energy level • Code: – 1, 2, 3, 4, 5, 6, 7, 8
• A charged particle • Cations: positive charged ion – “+” oxidation number • Anions: negative charged ion – “—” oxidation number IONS
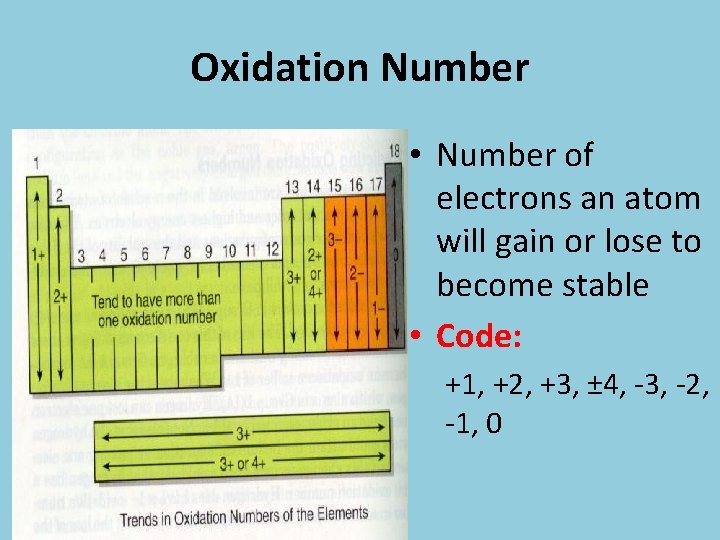
Oxidation Number • Number of electrons an atom will gain or lose to become stable • Code: +1, +2, +3, ± 4, -3, -2, -1, 0
Chemical Bond • Is the force that holds atoms or ions together • Three Types – Ionic – Covalent – Metallic
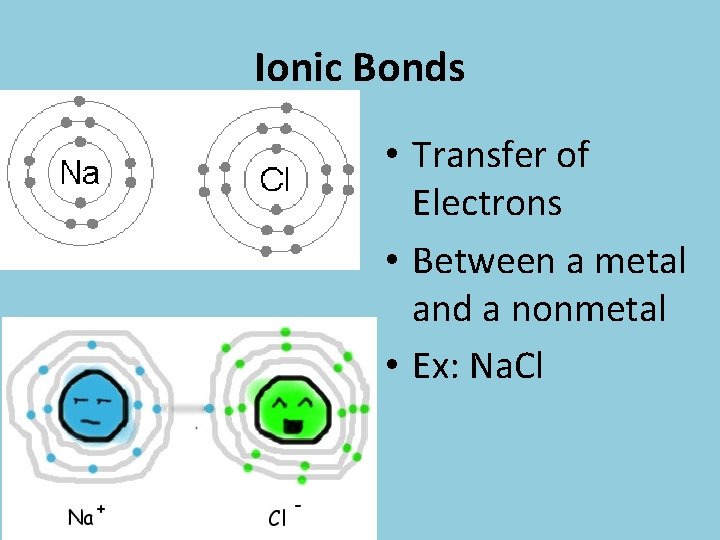
Ionic Bonds • Transfer of Electrons • Between a metal and a nonmetal • Ex: Na. Cl
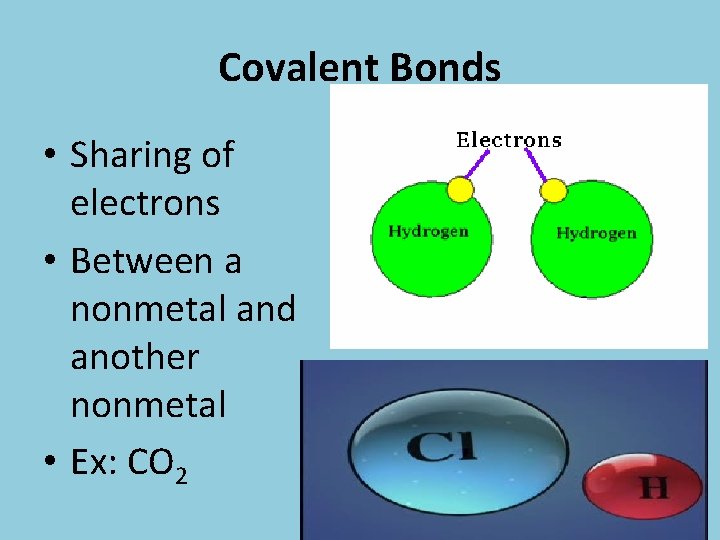
Covalent Bonds • Sharing of electrons • Between a nonmetal and another nonmetal • Ex: CO 2
Metallic Bonds • Sharing of electrons • Between a metal and another metal • Ex: Alloys
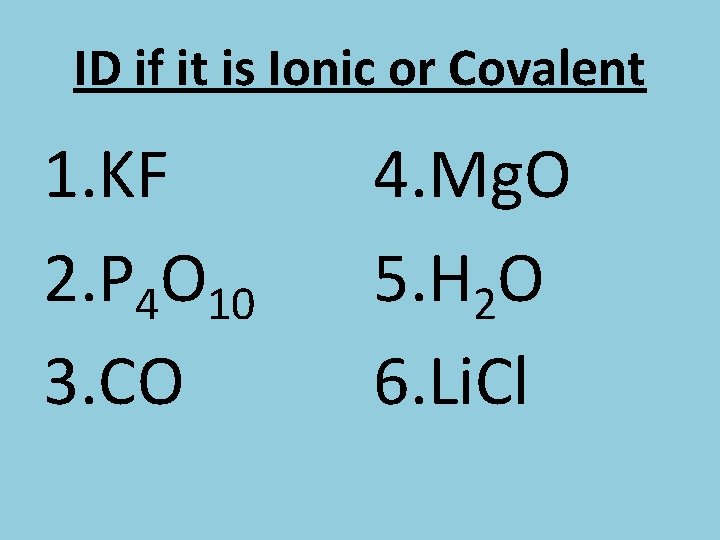
ID if it is Ionic or Covalent 1. KF 2. P 4 O 10 3. CO 4. Mg. O 5. H 2 O 6. Li. Cl
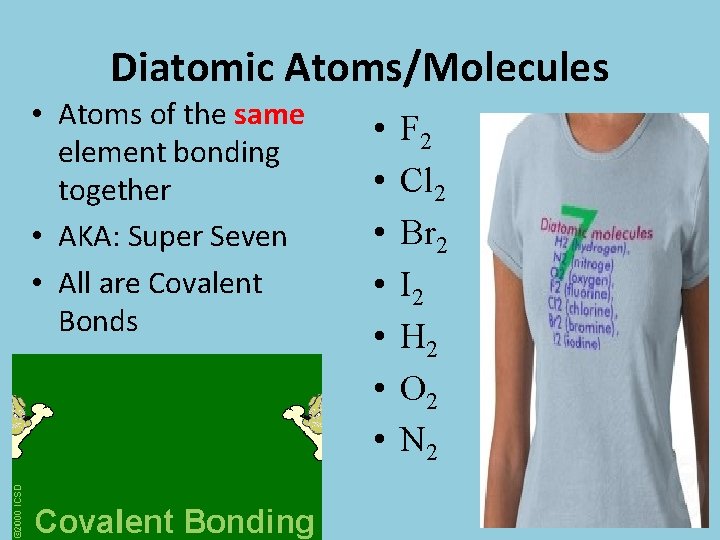
Diatomic Atoms/Molecules • Atoms of the same element bonding together • AKA: Super Seven • All are Covalent Bonds • • F 2 Cl 2 Br 2 I 2 H 2 O 2 N 2

End of Day One
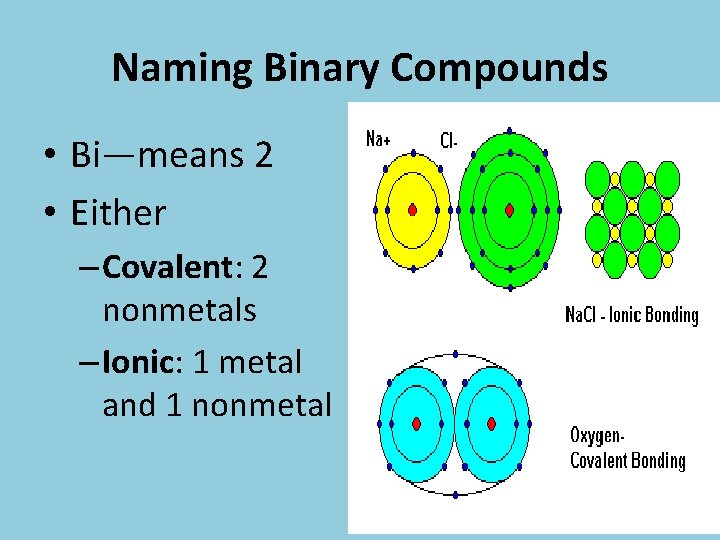
Naming Binary Compounds • Bi—means 2 • Either – Covalent: 2 nonmetals – Ionic: 1 metal and 1 nonmetal
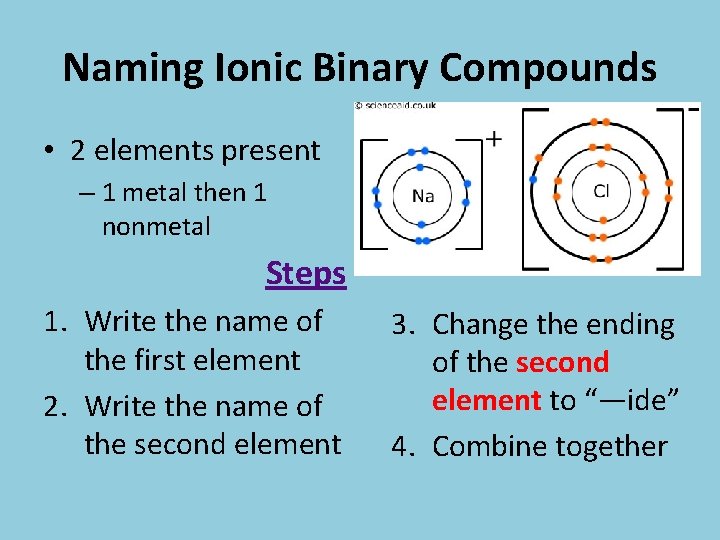
Naming Ionic Binary Compounds • 2 elements present – 1 metal then 1 nonmetal Steps 1. Write the name of the first element 2. Write the name of the second element 3. Change the ending of the second element to “—ide” 4. Combine together
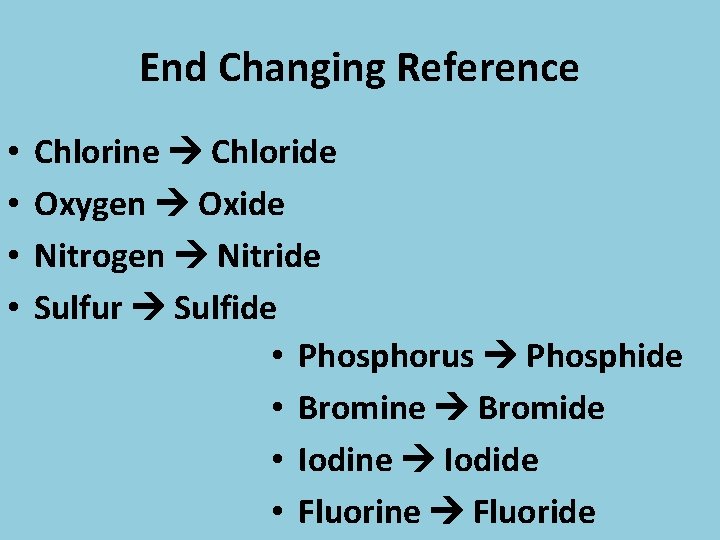
End Changing Reference • • Chlorine Chloride Oxygen Oxide Nitrogen Nitride Sulfur Sulfide • Phosphorus Phosphide • Bromine Bromide • Iodine Iodide • Fluorine Fluoride
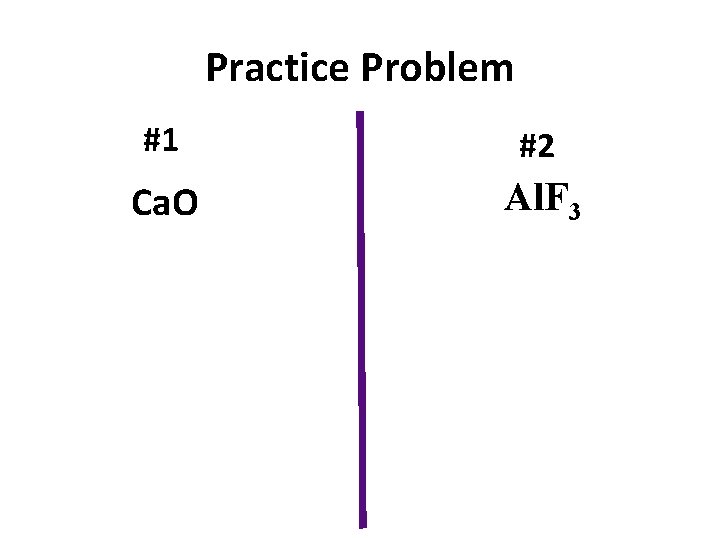
Practice Problem #1 #2 Ca. O Al. F 3
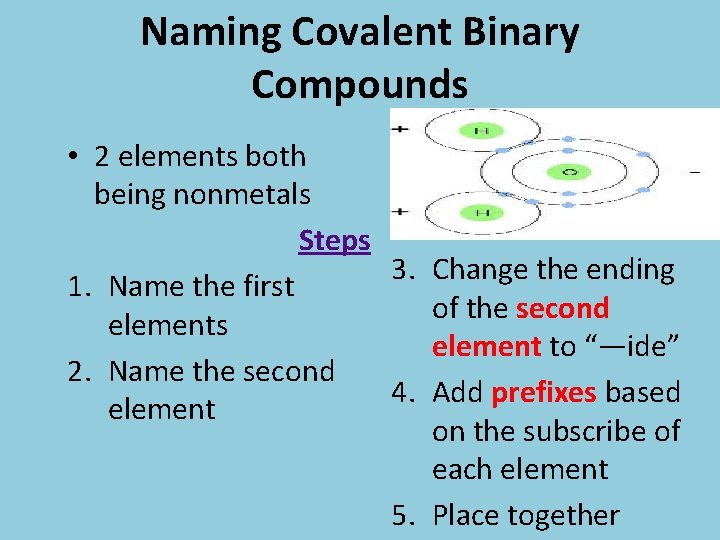
Naming Covalent Binary Compounds • 2 elements both being nonmetals Steps 3. Change the ending 1. Name the first of the second elements element to “—ide” 2. Name the second 4. Add prefixes based element on the subscribe of each element 5. Place together
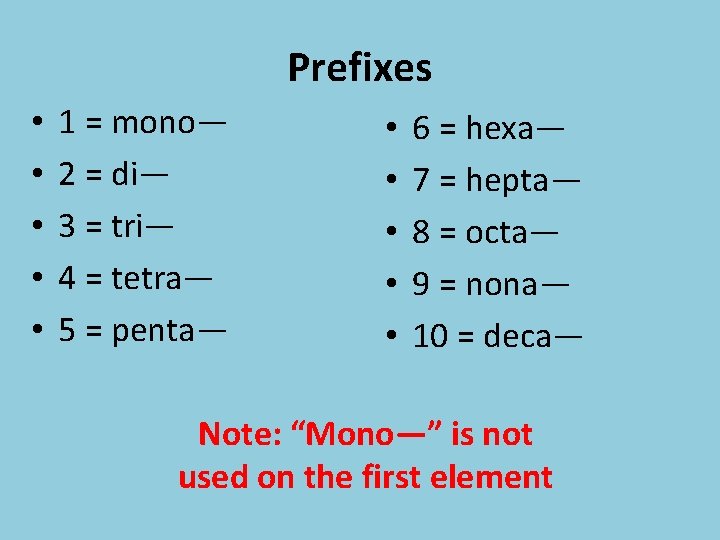
Prefixes • • • 1 = mono— 2 = di— 3 = tri— 4 = tetra— 5 = penta— • • • 6 = hexa— 7 = hepta— 8 = octa— 9 = nona— 10 = deca— Note: “Mono—” is not used on the first element
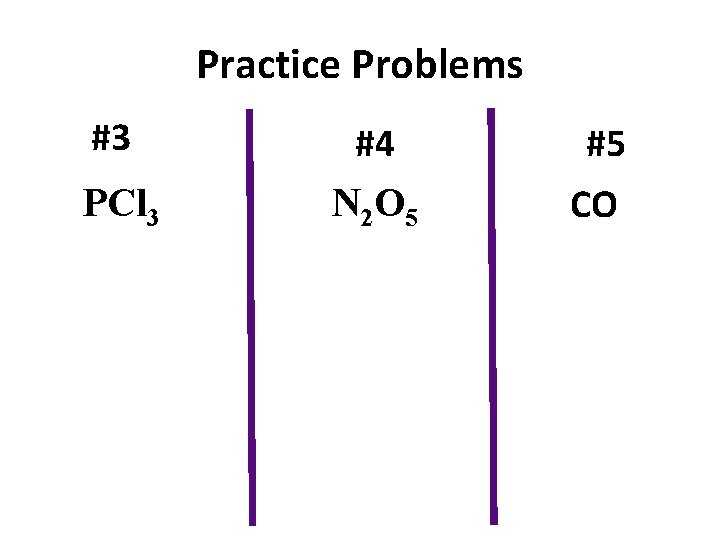
Practice Problems #3 PCl 3 #4 N 2 O 5 #5 CO

End of Day Two
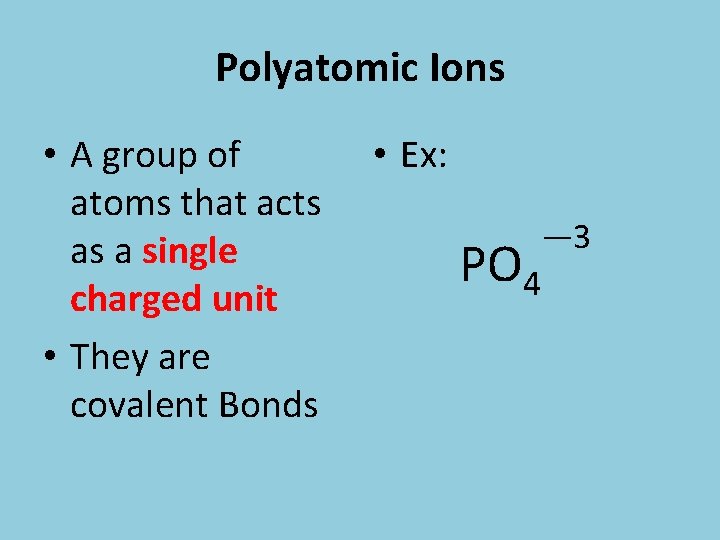
Polyatomic Ions • A group of atoms that acts as a single charged unit • They are covalent Bonds • Ex: PO 4 — 3
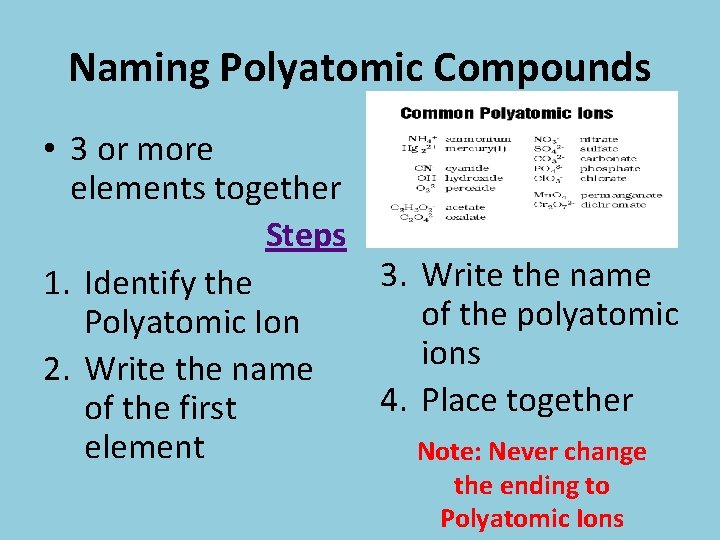
Naming Polyatomic Compounds • 3 or more elements together Steps 3. Write the name 1. Identify the of the polyatomic Polyatomic Ion ions 2. Write the name 4. Place together of the first element Note: Never change the ending to Polyatomic Ions
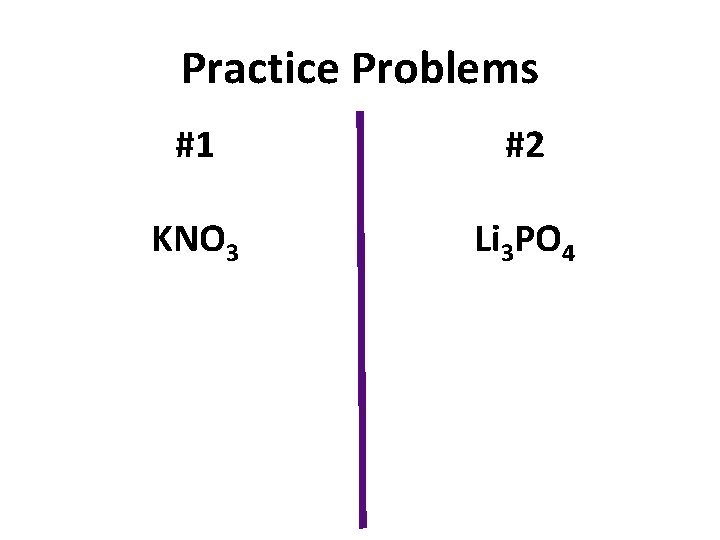
Practice Problems #1 #2 KNO 3 Li 3 PO 4
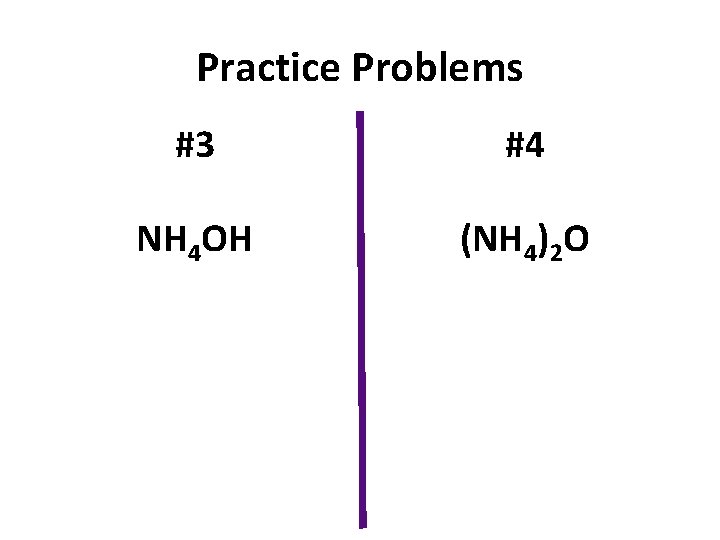
Practice Problems #3 #4 NH 4 OH (NH 4)2 O

End of Day Three

Writing Binary Formulas
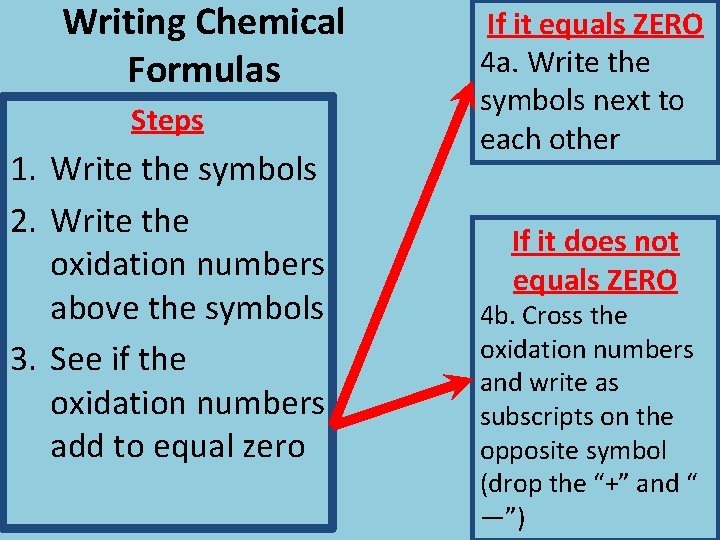
Writing Chemical Formulas Steps 1. Write the symbols 2. Write the oxidation numbers above the symbols 3. See if the oxidation numbers add to equal zero If it equals ZERO 4 a. Write the symbols next to each other If it does not equals ZERO 4 b. Cross the oxidation numbers and write as subscripts on the opposite symbol (drop the “+” and “ —”)
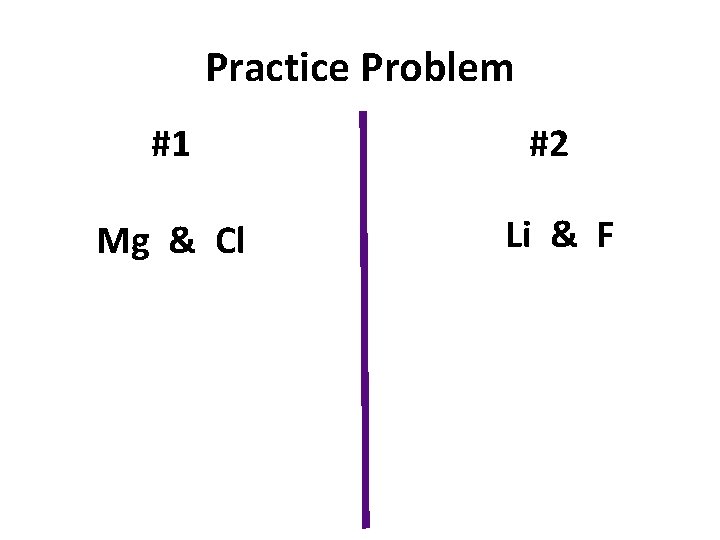
Practice Problem #1 #2 Mg & Cl Li & F
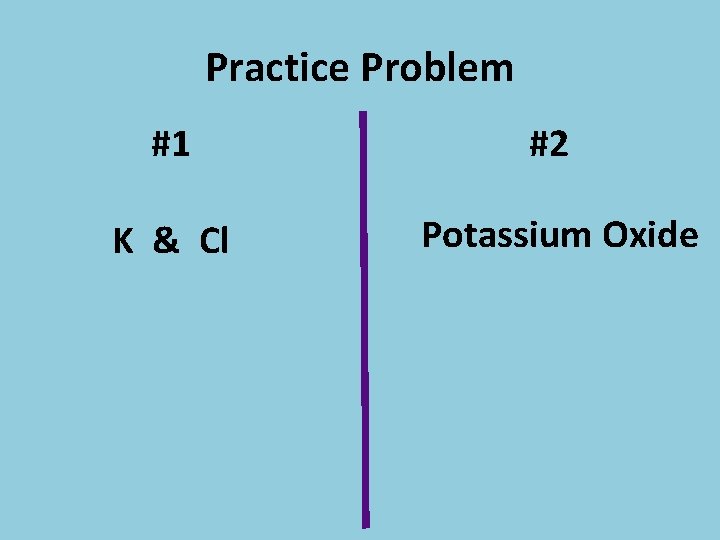
Practice Problem #1 #2 K & Cl Potassium Oxide

End of Day Four

Writing Formulas with Polyatomic Ions

Special Notes • If a coefficient of 2 or 3 is being applied to a polyatomic ion, parentheses goes around the polyatomic ion and the subscript is placed outside to the right
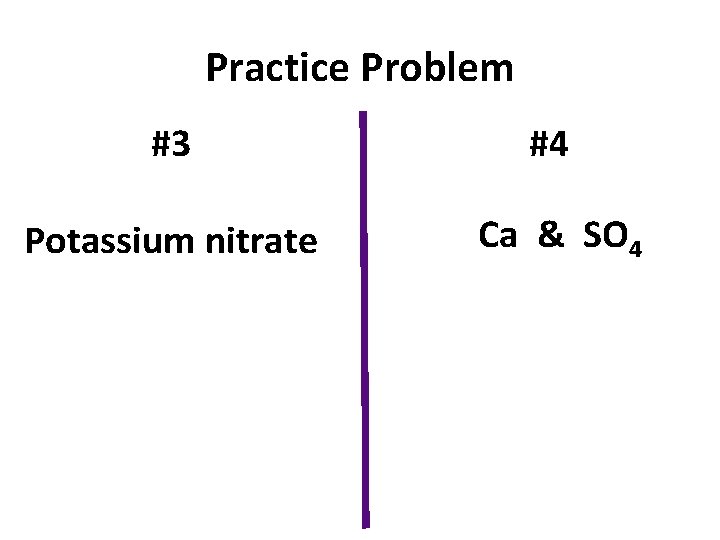
Practice Problem #3 #4 Potassium nitrate Ca & SO 4
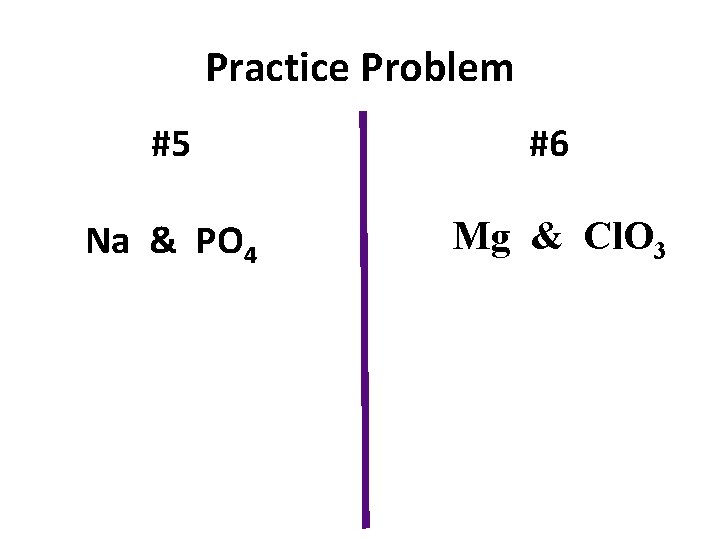
Practice Problem #5 #6 Na & PO 4 Mg & Cl. O 3
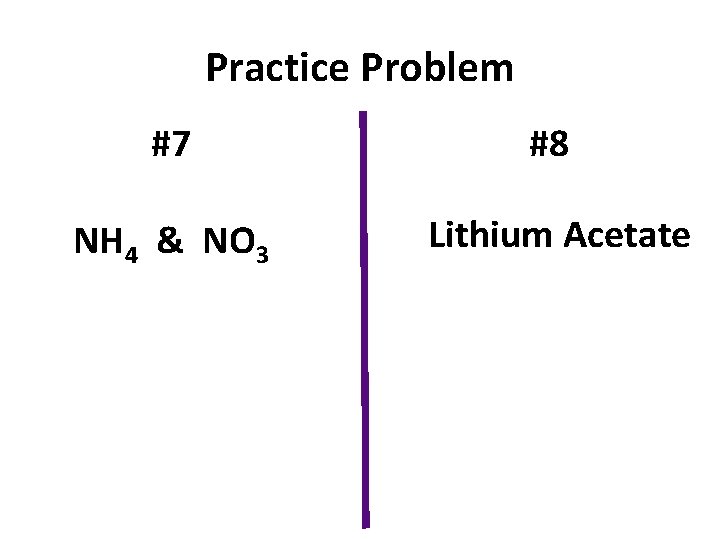
Practice Problem #7 #8 NH 4 & NO 3 Lithium Acetate

End of the Unit
- Slides: 37