Chemical Bonding Molecular Geometry How is molecular geometry
Chemical Bonding: Molecular Geometry
How is molecular geometry determined?
With VSEPR Theory! VSEPR Theory is the idea that electron groups repel one another. V = Valence S = Shell E = Electron P = Pair R = Repulsion
What is an electron group? An electron group can be defined as a lone pair, single bond, double bond, or triple bond.
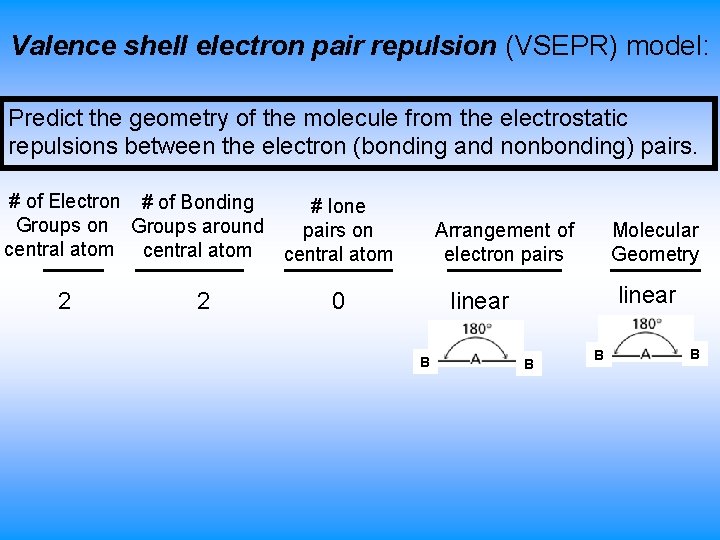
Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. # of Electron # of Bonding # lone Groups on Groups around pairs on central atom 2 2 Arrangement of electron pairs 0 Molecular Geometry linear B B
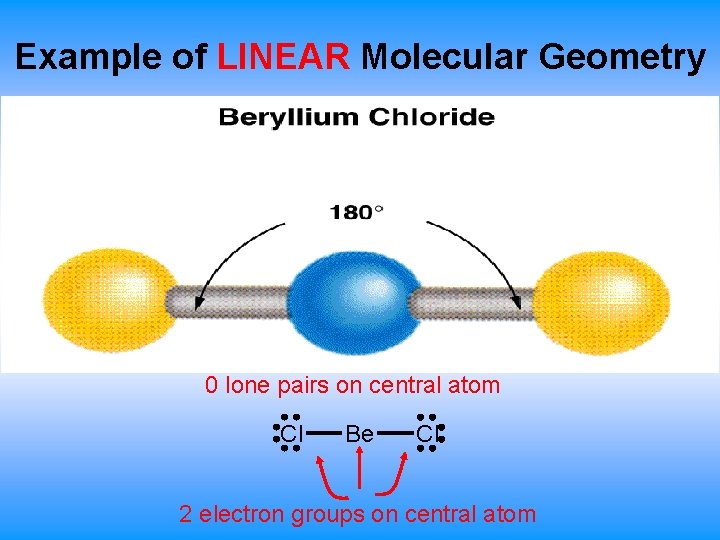
Example of LINEAR Molecular Geometry 0 lone pairs on central atom Cl Be Cl 2 electron groups on central atom
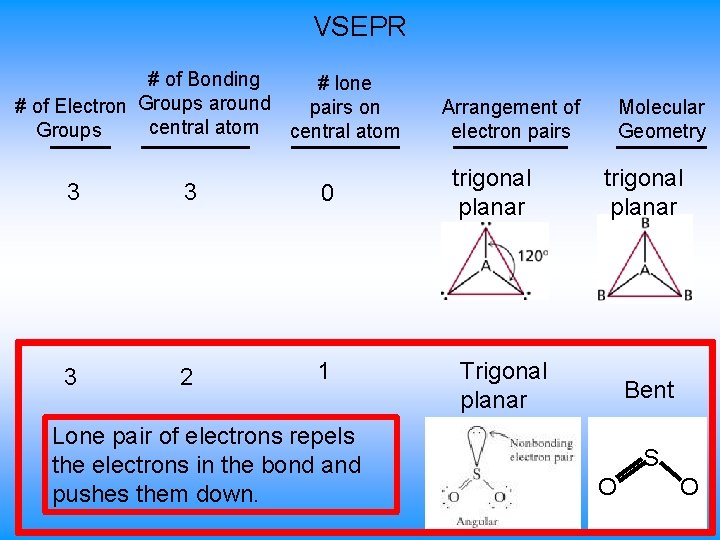
VSEPR # of Bonding # lone # of Electron Groups around pairs on central atom Groups central atom 3 3 0 3 2 1 Lone pair of electrons repels the electrons in the bond and pushes them down. Arrangement of electron pairs trigonal planar Molecular Geometry trigonal planar Trigonal planar Bent S O O
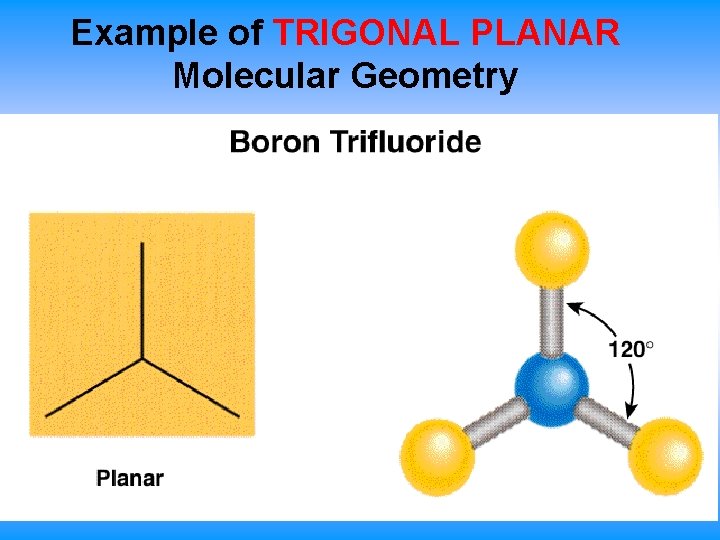
Example of TRIGONAL PLANAR Molecular Geometry
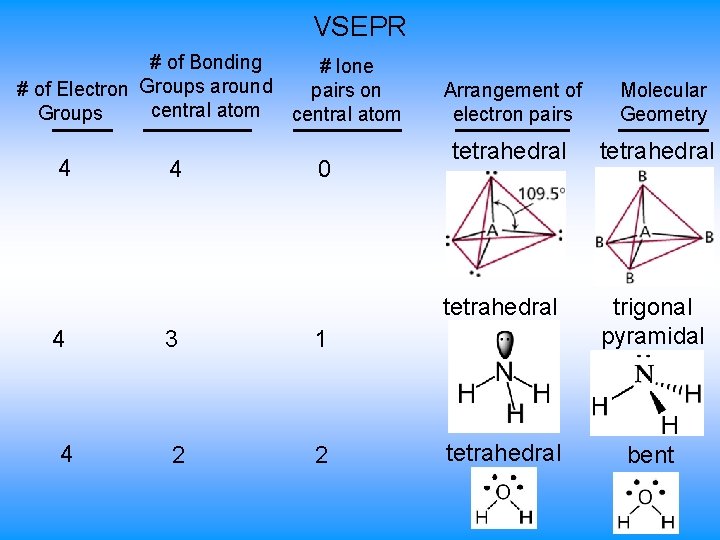
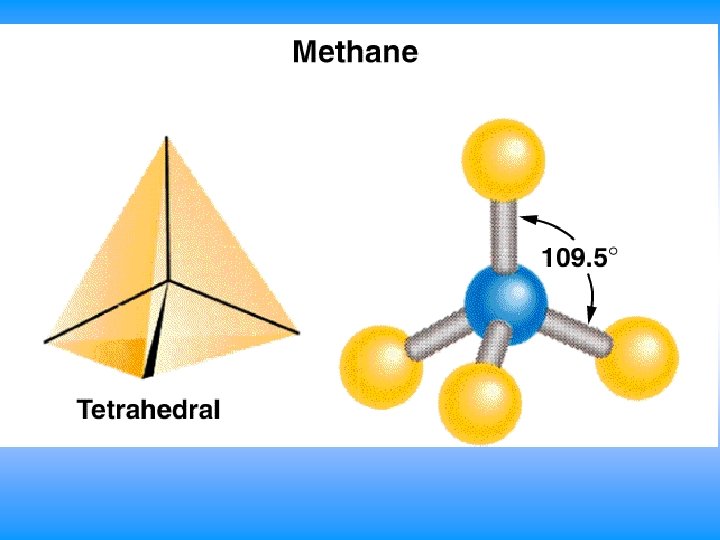
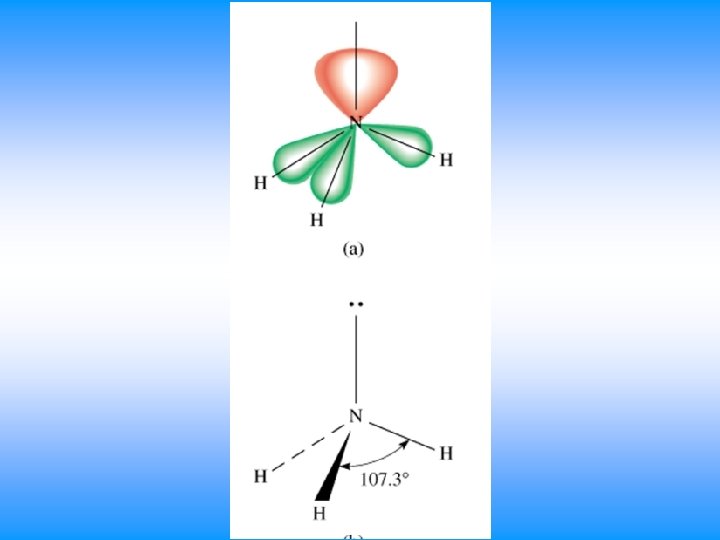
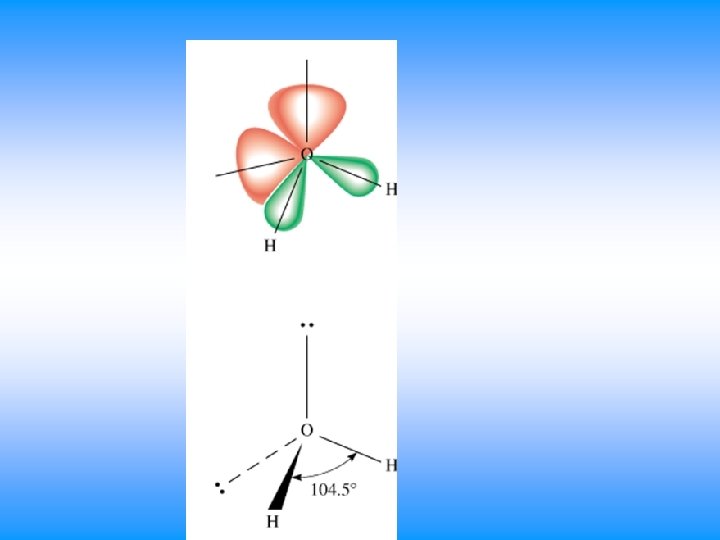
VSEPR # of Bonding # lone # of Electron Groups around pairs on central atom Groups central atom 4 4 3 2 0 Arrangement of electron pairs tetrahedral trigonal pyramidal tetrahedral bent 1 2 Molecular Geometry
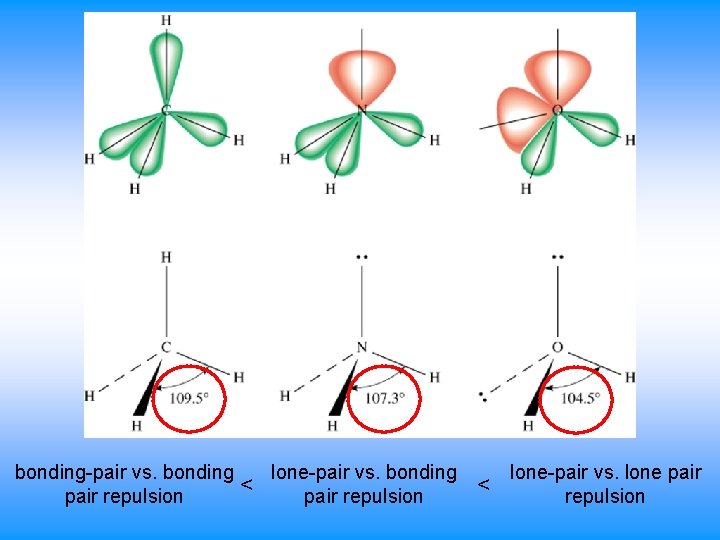
lone-pair vs. bonding-pair vs. bonding < pair repulsion < lone-pair vs. lone pair repulsion
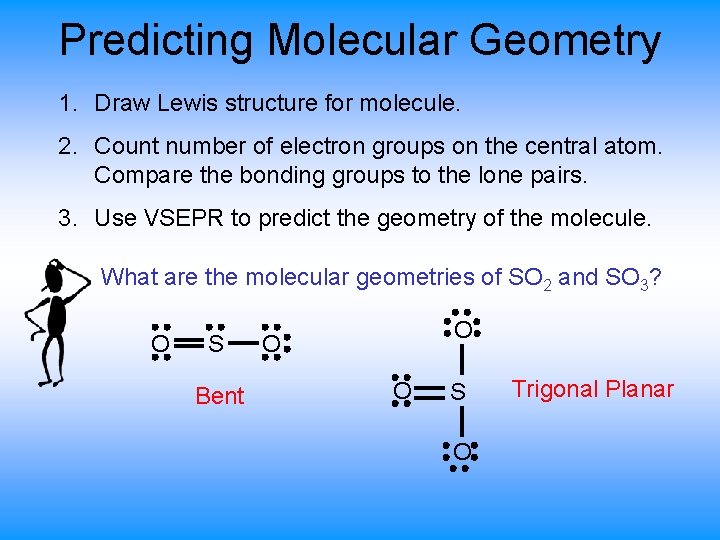
Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of electron groups on the central atom. Compare the bonding groups to the lone pairs. 3. Use VSEPR to predict the geometry of the molecule. What are the molecular geometries of SO 2 and SO 3? O S Bent O O O S O Trigonal Planar

Team Huddle- Review • In teams of 4 - lab groups • 1 white board per team, 1 marker per team • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

CO 2 • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

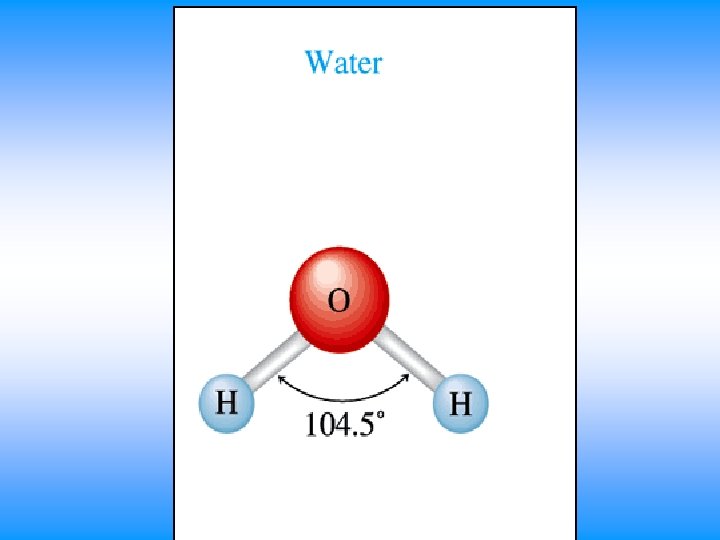
H 2 O • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

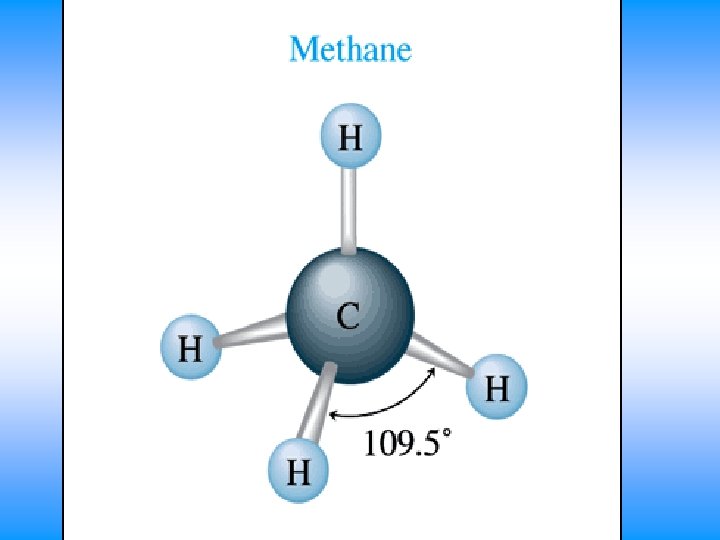
CH 4 • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

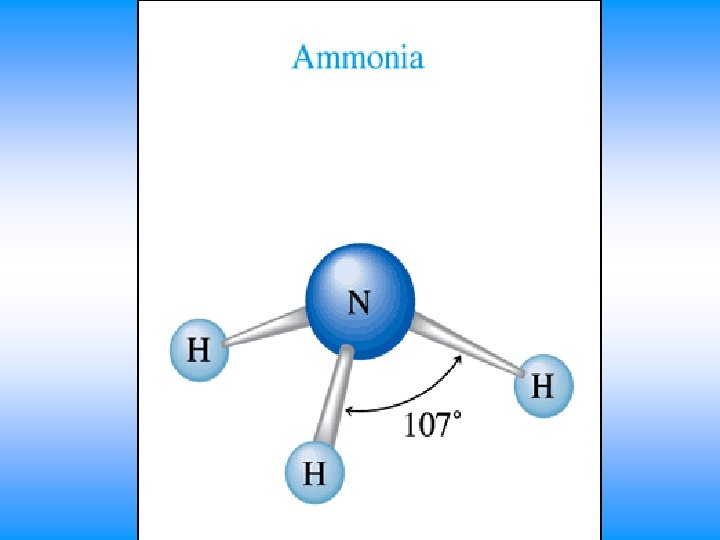
NH 3 • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

Al. H 3 • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

OH *CHALLENGE! has 1 extra electron • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

NH 4 +1 *CHALLENGE! has 1 extra electron • You will have 60 seconds to work with your team to determine: – Draw Lewis Dot Structure – Name of shape – State how many domains on central atom – Label bond angles

Practice- pages 18 & 20 Whatever you do not finish in class will be due 12/12! *There will be a quiz on 12/12 Learning Targets #1 -7
- Slides: 26