Chemical Bonding LETS REVIEW How do atoms form

Chemical Bonding LET’S REVIEW…

How do atoms form chemical bonds? By gaining, losing, or sharing electrons Why do atoms form bonds? To attain a noble gas configuration

A + B AB When a bond formed = energy is released = reaction is exothermic

Breaking a Bond Absorbs Energy (endothermic)!
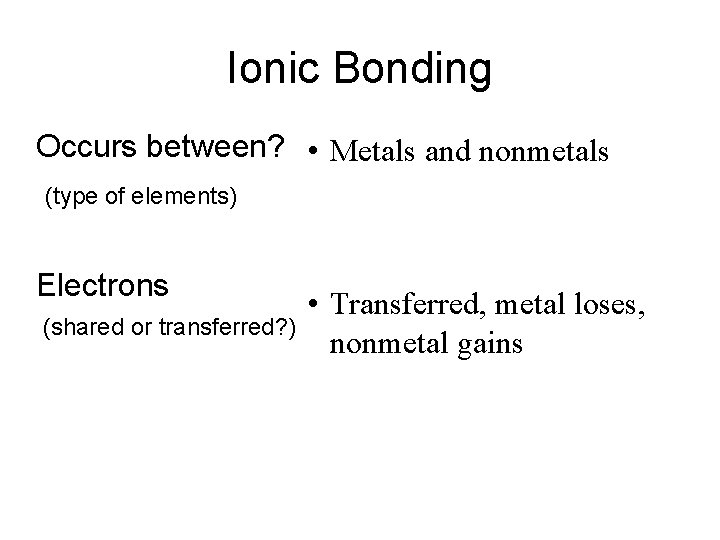
Ionic Bonding Occurs between? • Metals and nonmetals (type of elements) Electrons (shared or transferred? ) • Transferred, metal loses, nonmetal gains
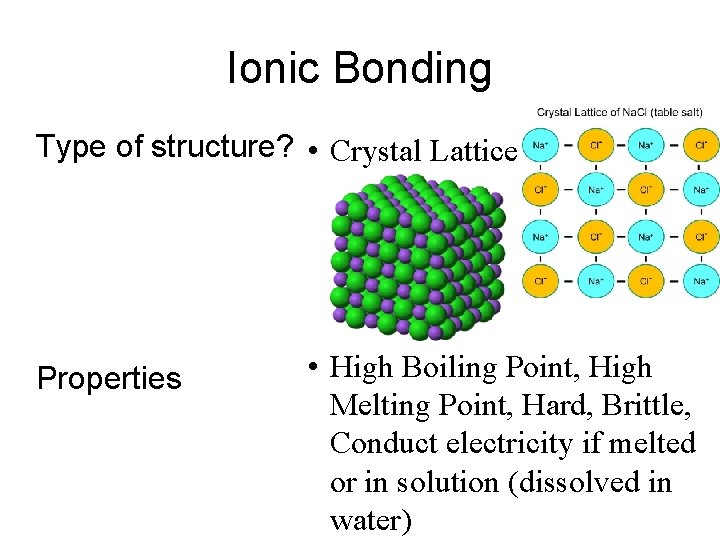
Ionic Bonding Type of structure? • Crystal Lattice Properties • High Boiling Point, High Melting Point, Hard, Brittle, Conduct electricity if melted or in solution (dissolved in water)
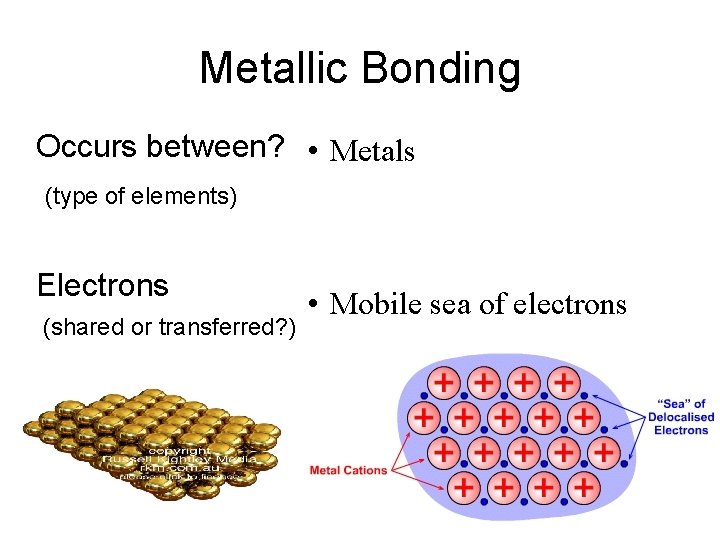
Metallic Bonding Occurs between? • Metals (type of elements) Electrons (shared or transferred? ) • Mobile sea of electrons
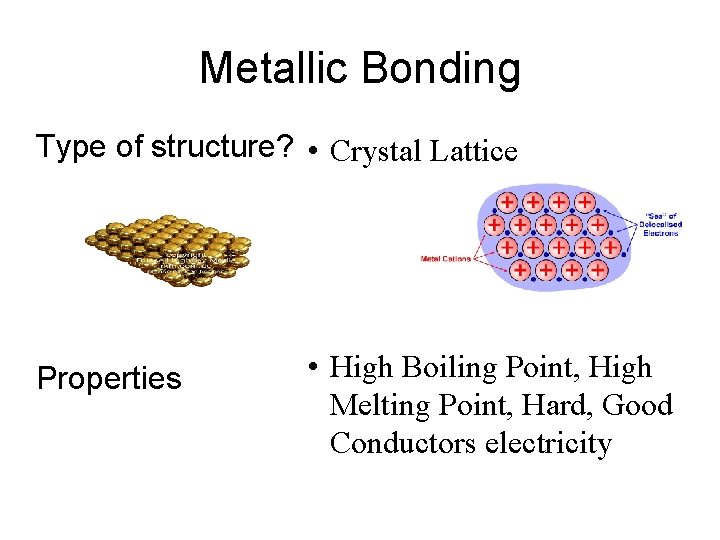
Metallic Bonding Type of structure? • Crystal Lattice Properties • High Boiling Point, High Melting Point, Hard, Good Conductors electricity
Covalent Bonding Occurs between? • Nonmetals only (type of elements) Electrons (shared or transferred? ) • shared
Covalent Bonding Type of structure? • Molecules – held together by IMF (intermolecular forces) Properties • Low Boiling Point, Low Melting Point, soft, poor conductors of electricity
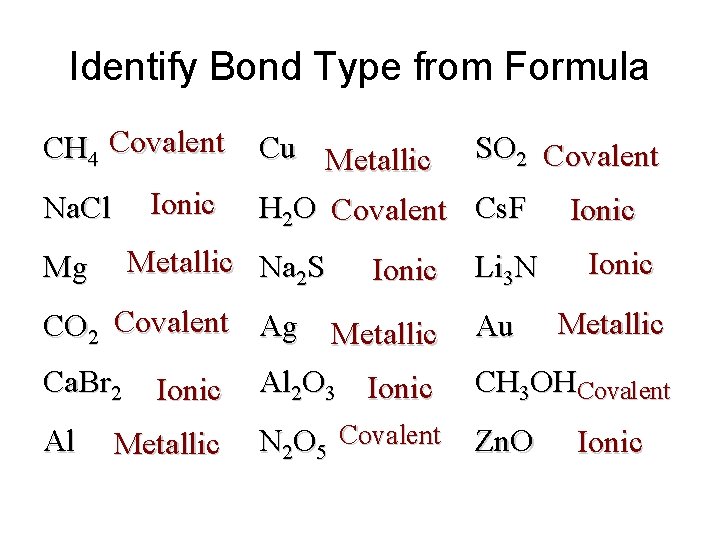
Identify Bond Type from Formula CH 4 Covalent Na. Cl Mg Ionic Cu Metallic SO 2 Covalent H 2 O Covalent Cs. F Ionic Metallic Na 2 S Ionic CO 2 Covalent Ag Metallic Ca. Br 2 Ionic Al 2 O 3 Ionic Al Metallic N 2 O 5 Covalent Li 3 N Au Ionic Metallic CH 3 OHCovalent Zn. O Ionic
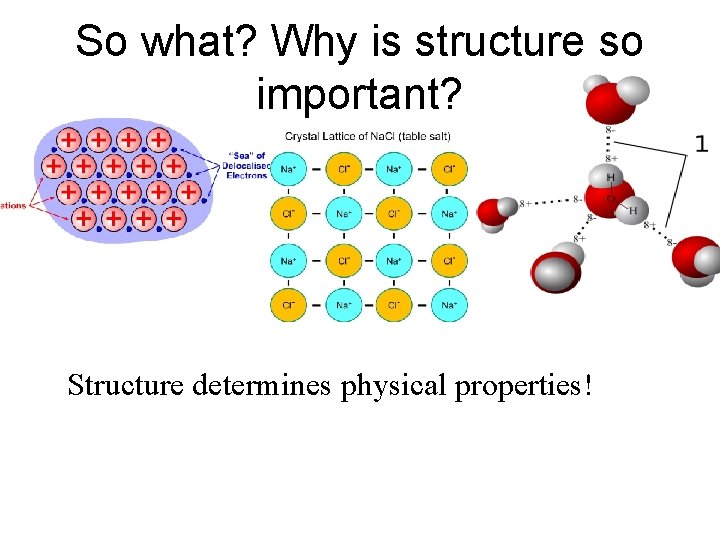
So what? Why is structure so important? Structure determines physical properties!
- Slides: 12