Chemical Bonding Lesson 1 Ionic Bonds Compounds Remember

Chemical Bonding Lesson 1 – Ionic Bonds & Compounds
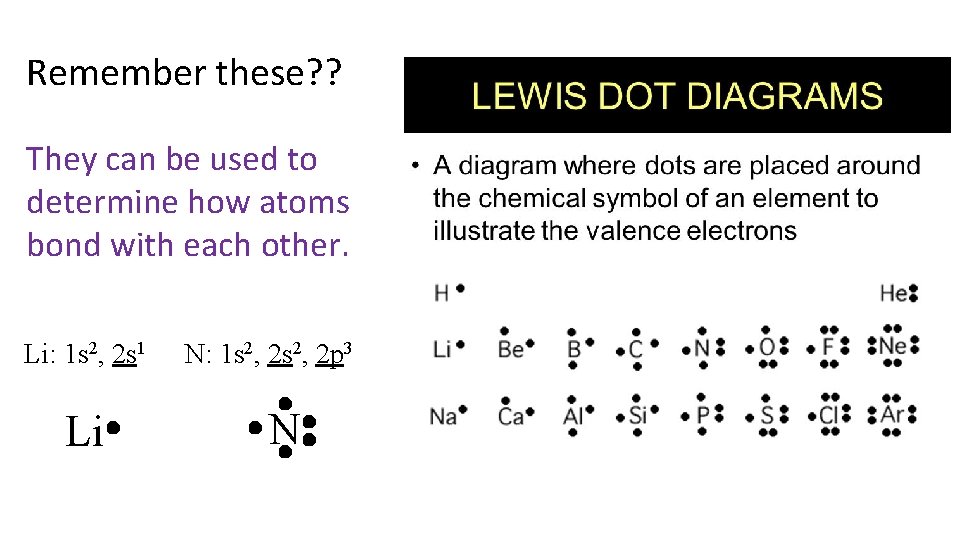
Remember these? ? They can be used to determine how atoms bond with each other. Li: 1 s 2, 2 s 1 Li N: 1 s 2, 2 p 3 N

The Octet Rule • Atoms are most stable when their outermost shell is full • Level 1 = 2 electrons • All other shells = 8 electrons • Noble gases have full outer shell so most do not bond with other atoms • Other atoms lose, gain, or share electrons to get a full outer shell
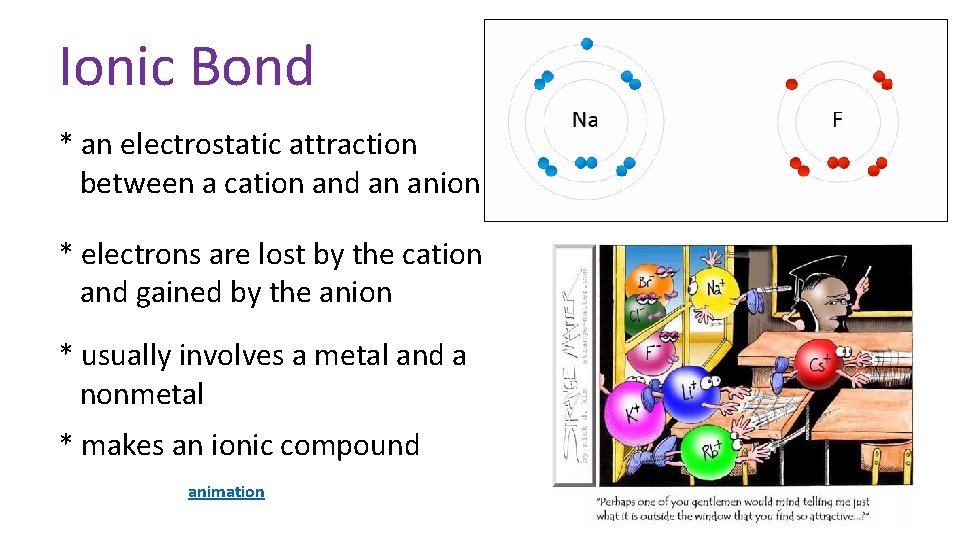
Ionic Bond * an electrostatic attraction between a cation and an anion * electrons are lost by the cation and gained by the anion * usually involves a metal and a nonmetal * makes an ionic compound animation
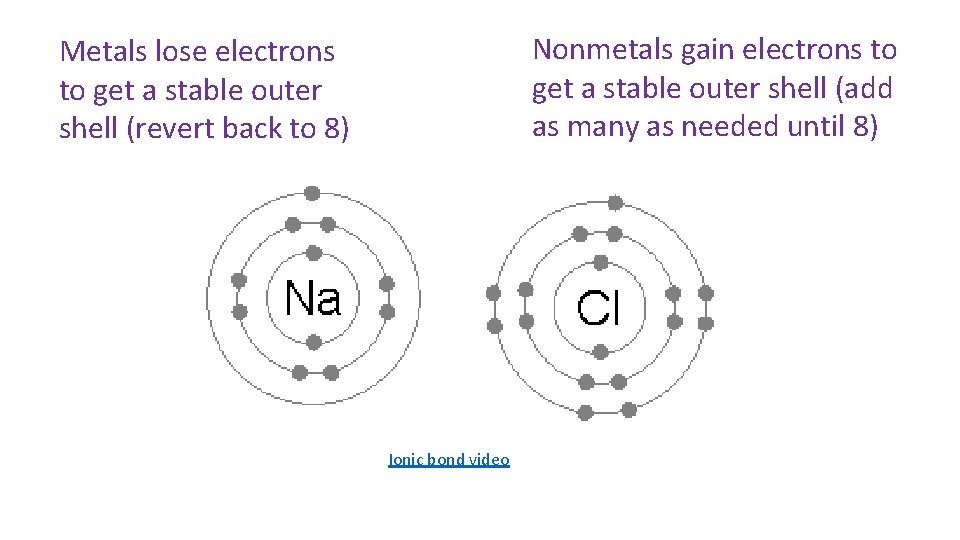
Nonmetals gain electrons to get a stable outer shell (add as many as needed until 8) Metals lose electrons to get a stable outer shell (revert back to 8) Ionic bond video
Properties of Ionic compounds Most have high melting points and high boiling points. ¡ Many dissolve in water (aqueous solution). ¡ Good conductors of electricity (electrolyte) if melted (molten) or dissolved. ¡ The greater the degree of ionization or dissociation the greater the conductivity of the solution
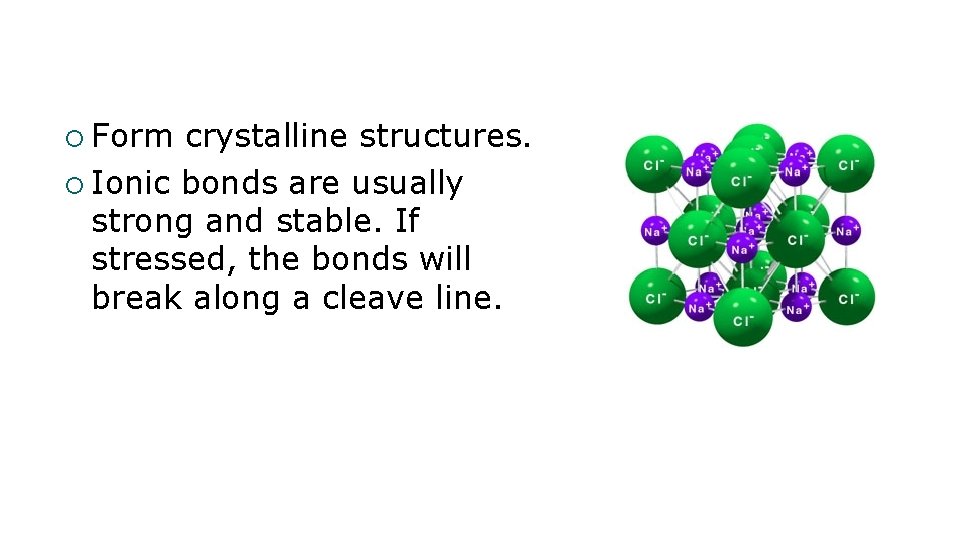
¡ Form crystalline structures. ¡ Ionic bonds are usually strong and stable. If stressed, the bonds will break along a cleave line.
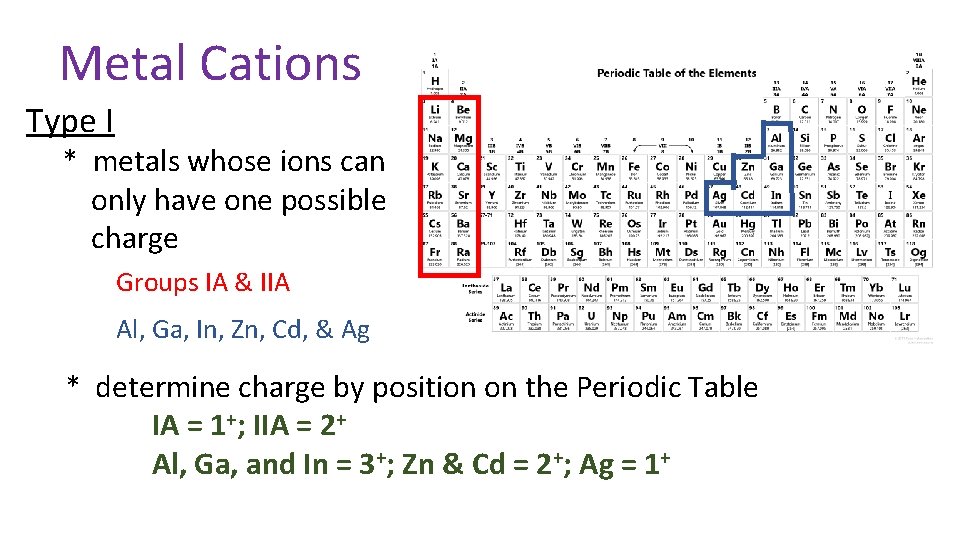
Metal Cations Type I * metals whose ions can only have one possible charge Groups IA & IIA Al, Ga, In, Zn, Cd, & Ag * determine charge by position on the Periodic Table IA = 1+; IIA = 2+ Al, Ga, and In = 3+; Zn & Cd = 2+; Ag = 1+
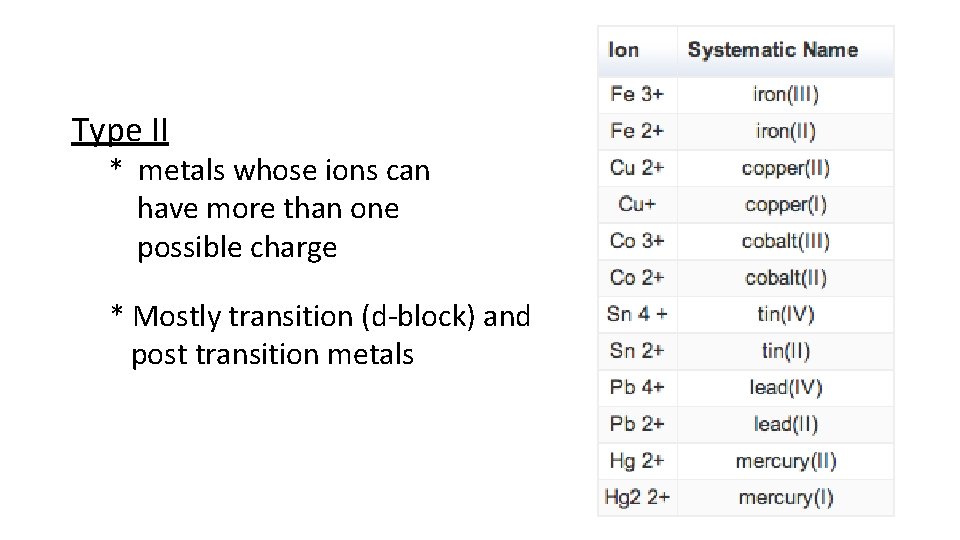
Type II * metals whose ions can have more than one possible charge * Mostly transition (d-block) and post transition metals

Polyatomic Ions * made of more than one atom. * groups of atoms that travel together—never apart. * The given charge is on the entire polyatomic ion—not on individual atoms within the polyatomic ion. A list can be found on page 7 of your reference packet. You are responsible for knowing: ammonium, cyanide, acetate, nitrite, hydroxide, carbonate, sulfite, and phosphate You will have a quiz on the names, symbols and charges of these polyatomic ions.

Naming Ionic Compounds * ALWAYS name the cation first and the anion second * Cation – metal or polyatomic ion name *** If the cation is a Type 2 metal, you must determine the charge of the metal and use that in the name*** * Anion – 1. if nonmetal change end of name to -ide 2. if polyatomic ion use the name

Name the following • KCl Potassium Chloride • Na. Cl Sodium Chloride • Mg. O Magnesium Oxide • Li 3 PO 4 Lithium Phosphate • NH 4 Cl Ammonium Chloride
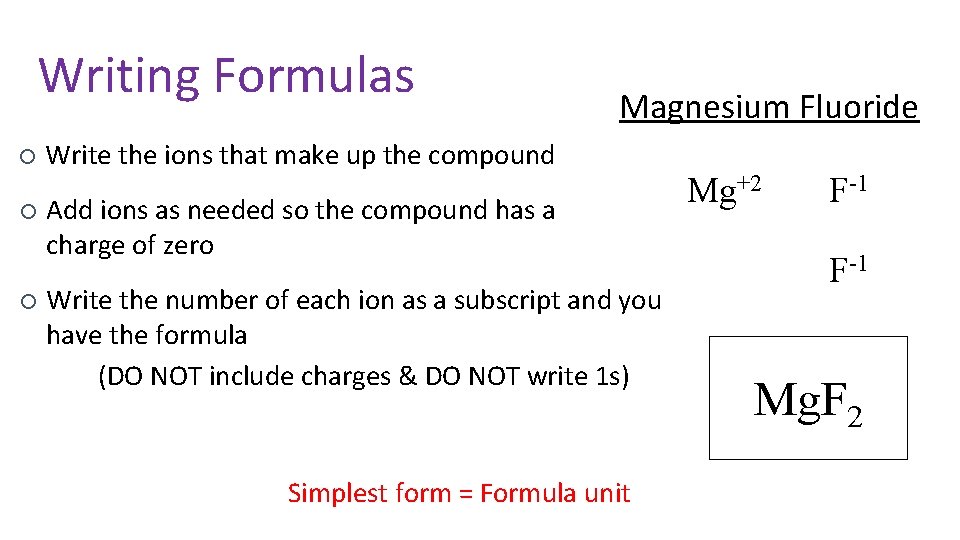
Writing Formulas ¡ ¡ ¡ Magnesium Fluoride Write the ions that make up the compound Add ions as needed so the compound has a charge of zero Write the number of each ion as a subscript and you have the formula (DO NOT include charges & DO NOT write 1 s) Simplest form = Formula unit Mg+2 F-1 Mg. F 2
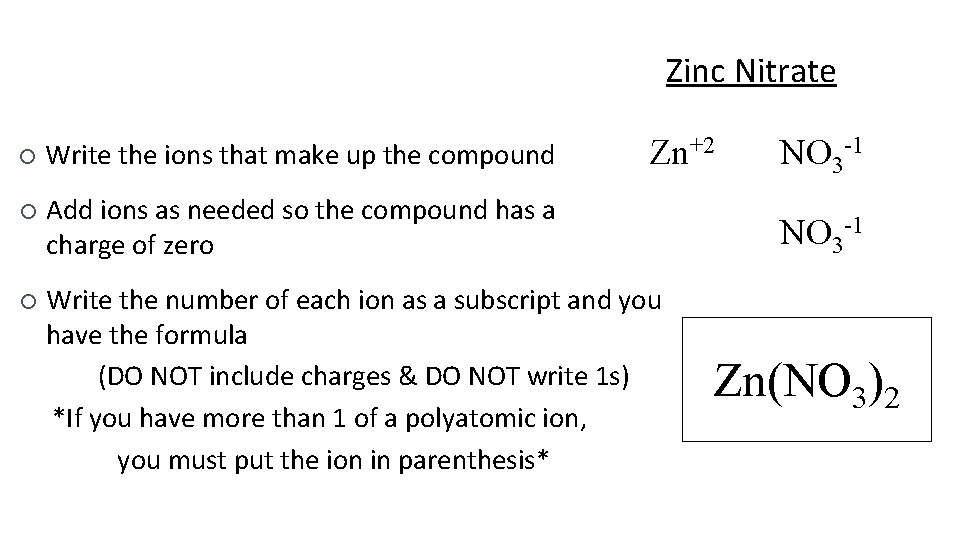
Zinc Nitrate Zn+2 ¡ Write the ions that make up the compound ¡ Add ions as needed so the compound has a charge of zero ¡ Write the number of each ion as a subscript and you have the formula (DO NOT include charges & DO NOT write 1 s) *If you have more than 1 of a polyatomic ion, you must put the ion in parenthesis* NO 3 -1 Zn(NO 3)2
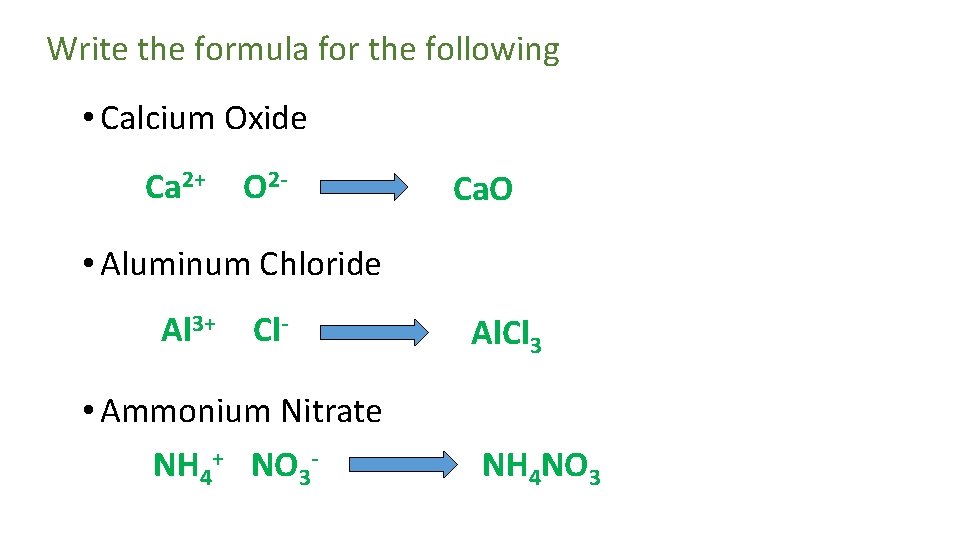
Write the formula for the following • Calcium Oxide Ca 2+ O 2 - Ca. O • Aluminum Chloride Al 3+ Cl- • Ammonium Nitrate NH 4+ NO 3 - Al. Cl 3 NH 4 NO 3
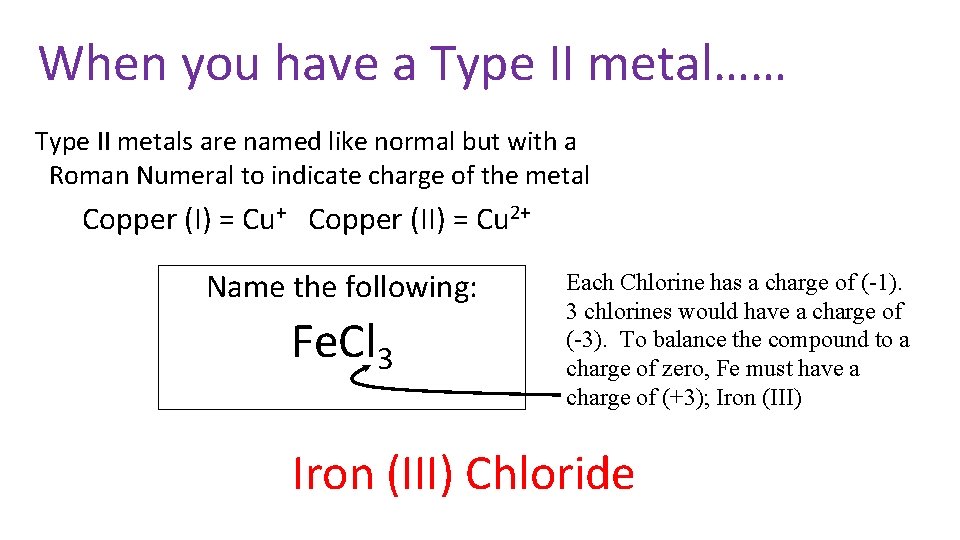
When you have a Type II metal…… Type II metals are named like normal but with a Roman Numeral to indicate charge of the metal Copper (I) = Cu+ Copper (II) = Cu 2+ Name the following: Fe. Cl 3 Each Chlorine has a charge of (-1). 3 chlorines would have a charge of (-3). To balance the compound to a charge of zero, Fe must have a charge of (+3); Iron (III) Chloride
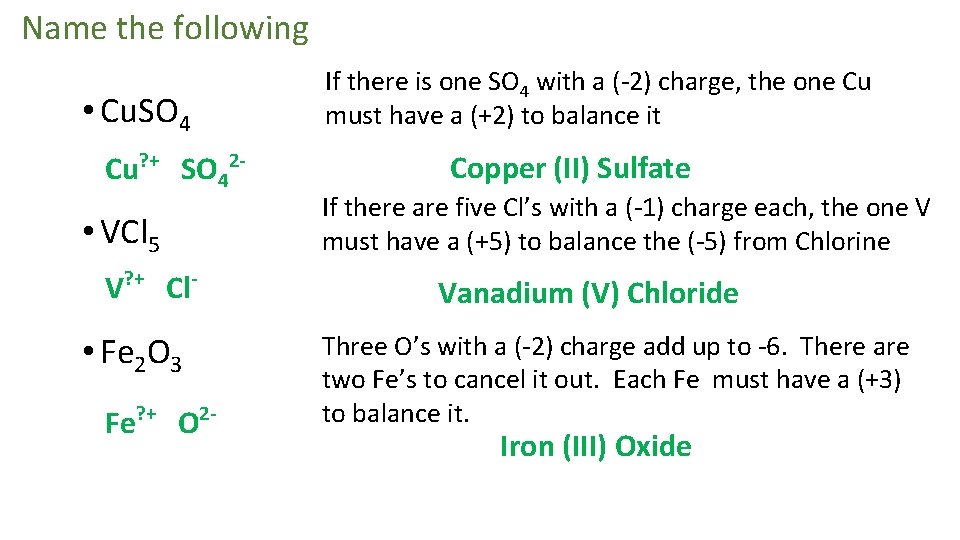
Name the following If there is one SO 4 with a (-2) charge, the one Cu must have a (+2) to balance it • Cu. SO 4 Cu? + SO 42 - • VCl 5 V? + Cl- Fe O If there are five Cl’s with a (-1) charge each, the one V must have a (+5) to balance the (-5) from Chlorine Vanadium (V) Chloride • Fe 2 O 3 ? + Copper (II) Sulfate 2 - Three O’s with a (-2) charge add up to -6. There are two Fe’s to cancel it out. Each Fe must have a (+3) to balance it. Iron (III) Oxide

- Slides: 18