Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride

Chemical BONDING IONIC Lewis Dot Diagrams

Sodium Chloride • This is the finished Lewis Dot Structure How did we get here? +1 [Na] [ Cl ] -1

Practice Dot diagrams & formulas • Lithium fluoride • Magnesium oxide • Calcium chloride • Potassium hydride
Drawing molecules using Lewis Dot Structures Remember: atoms are sharing eto complete their outer shell!
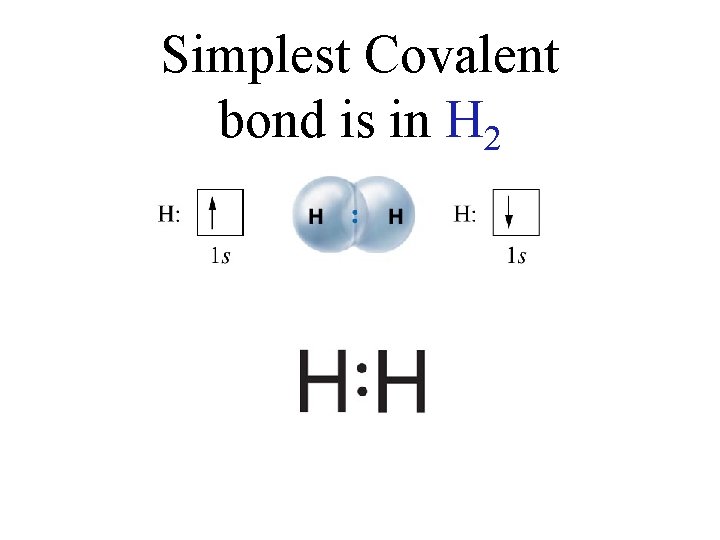
Simplest Covalent bond is in H 2

F 2 • Valence orbital notation for each atom? • Lewis Dot diagram for molecule?
Multiple Covalent Bonds • O 2 • Valence orbital notation for each atom? – The overlap results in a DOUBLE BOND • Lewis Dot Structure?
Multiple Covalent Bonds • N 2 • Valence orbital notation for each atom? – The overlap results in a TRIPLE BOND • Lewis Dot Structure?
Use Pencil • Step 1 – count total valence e- involved; this is your target number. No more! No less! • Step 2 – connect the atoms with single bonds **you may need to reconfigure the dots later… ahhhhh pencil • Step 3 – complete valence shells to make the atoms stable.

Let’s Practice! • make sure all the atoms are complete and stable. Just use dots…no x HCl CH 4 NH 3

Draw the Lewis Dot Diagram for polyatomic ions • Same thing! • Just add or subtract electrons based on the charge, enclose in a giant bracket and write its charge. REMEMBER! A negative charge means it has extra electrons! Phosphate

The End
- Slides: 13