Chemical Bonding Ionic Covalent Metallic Compounds Bonds p
Chemical Bonding Ionic, Covalent, & Metallic Compounds

Bonds p Chemical Bond n p 3 attractive force between atoms or ions that binds them together as a unit Types of Bonds p Ionic p Covalent p Metallic
Ionic Bonds p What types of atoms typically form ionic bonds? p How are ionic bonds formed and what type of structure do they typically create? p What are the typical properties of ionic substances?
Forming Ionic Bonds formed when an electron is transferred from a metal to a nonmetal p Bond between a cation and an anion p Give and take p p Reaction between sodium and chlorine: http: //www. visionlearning. com/library/flas h_viewer. php? oid=1349&mid=55
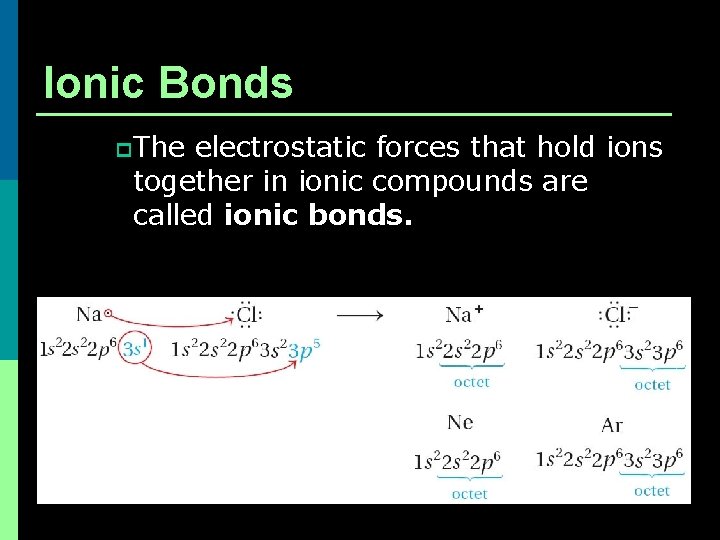
Ionic Bonds p The electrostatic forces that hold ions together in ionic compounds are called ionic bonds.
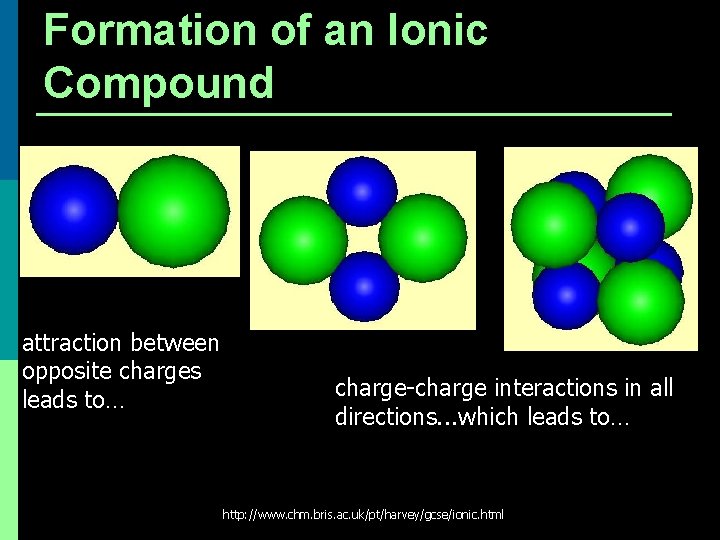
Formation of an Ionic Compound attraction between opposite charges leads to… charge-charge interactions in all directions. . . which leads to… http: //www. chm. bris. ac. uk/pt/harvey/gcse/ionic. html
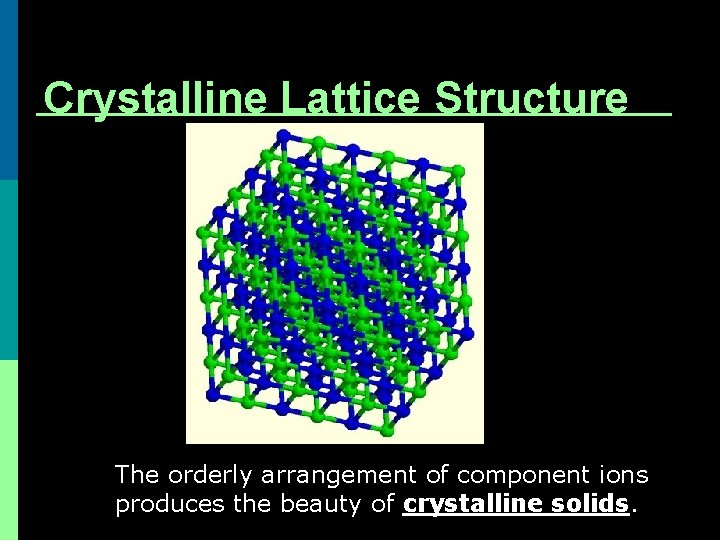
Crystalline Lattice Structure The orderly arrangement of component ions produces the beauty of crystalline solids.

7. 2 Properties of Ionic Compounds § Ionic compounds can conduct an electric current when melted or dissolved in water.

Covalent Bonds p What types of atoms typically form covalent bonds? p How are covalent bonds formed and what type of structure do they create? p What are the typical properties of covalent substances?
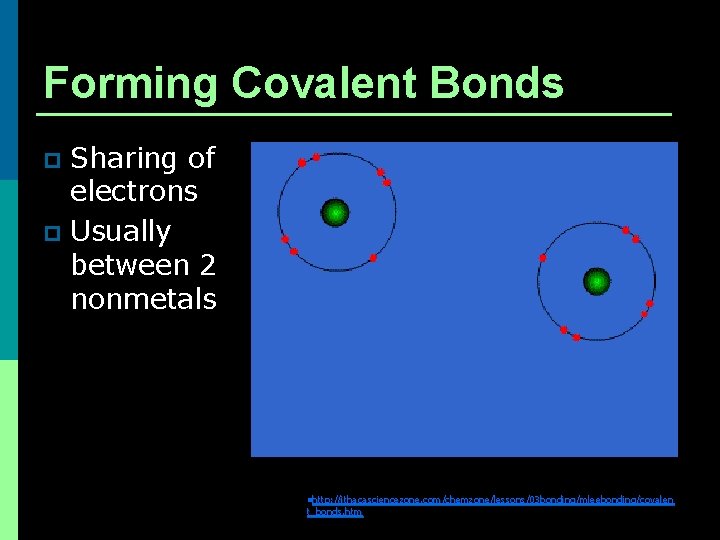
Forming Covalent Bonds Sharing of electrons p Usually between 2 nonmetals p nhttp: //ithacasciencezone. com/chemzone/lessons/03 bonding/mleebonding/covalen t_bonds. htm
Molecules p. A molecule is a neutral group of atoms joined together by covalent bonds. Air contains oxygen molecules. p. A diatomic molecule is a molecule consisting of two atoms. An oxygen molecule is a diatomic molecule.
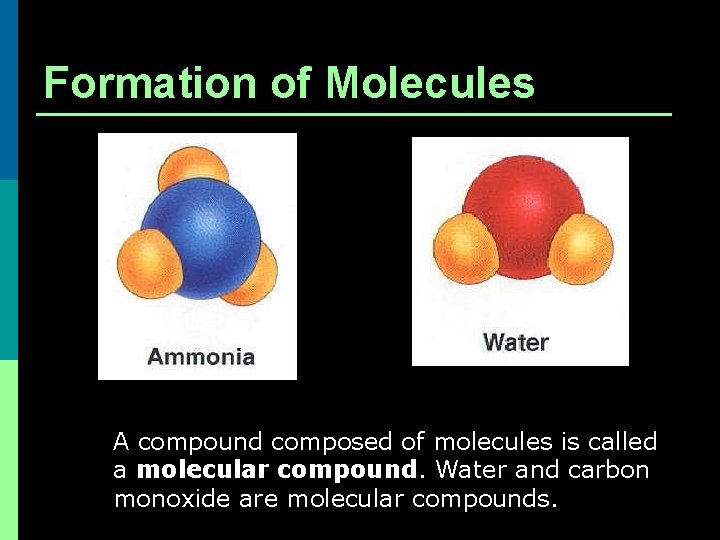
Formation of Molecules A compound composed of molecules is called a molecular compound. Water and carbon monoxide are molecular compounds.
Properties of Molecular Compounds n Molecular compounds tend to have relatively lower melting and boiling points than ionic compounds. n Formation of Methane – you tube

Metallic Bonds p What types of atoms typically form metallic bonds? p How are metallic bonds formed and what type of structure do they create? p What are the typical properties of metallic substances?
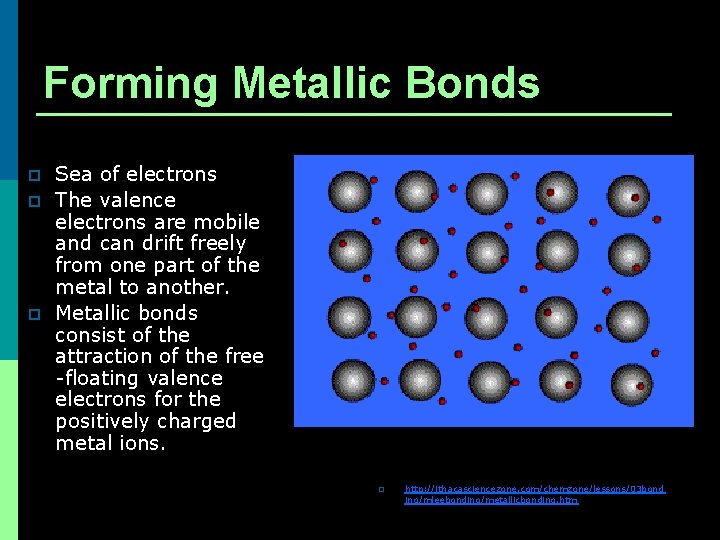
Forming Metallic Bonds p p p Sea of electrons The valence electrons are mobile and can drift freely from one part of the metal to another. Metallic bonds consist of the attraction of the free -floating valence electrons for the positively charged metal ions. p http: //ithacasciencezone. com/chemzone/lessons/03 bond ing/mleebonding/metallicbonding. htm
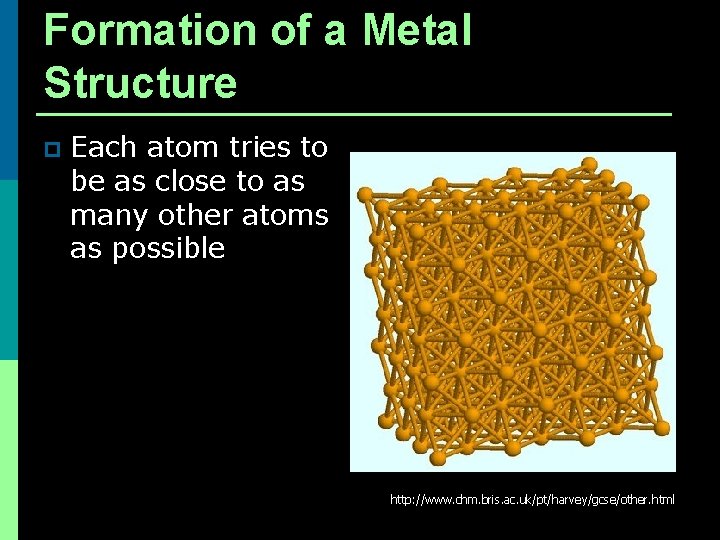
Formation of a Metal Structure p Each atom tries to be as close to as many other atoms as possible http: //www. chm. bris. ac. uk/pt/harvey/gcse/other. html
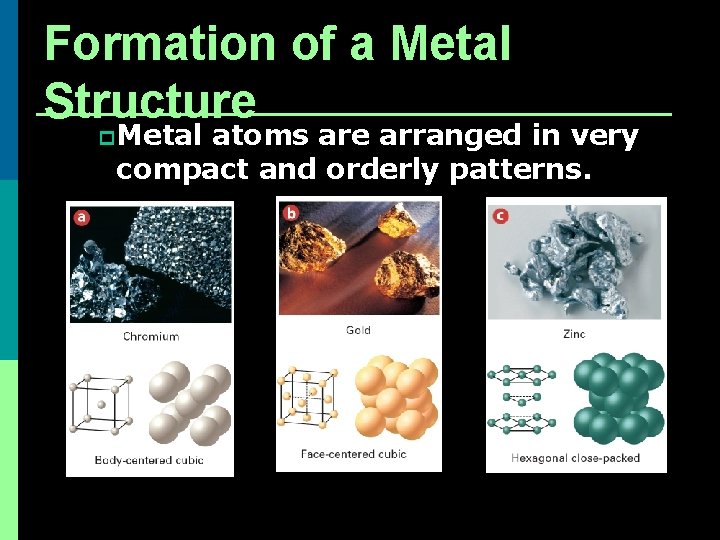
Formation of a Metal Structure p Metal atoms are arranged in very compact and orderly patterns.

Alloys ALLOYS are mixtures composed of two or more metals p Two types of alloys: p n INTERSTITIAL - smaller atoms can fit into the spaces between larger atoms SUBSTITUTIONAL - atoms within the alloy are about the same size and simply replace each other in the crystal n Ex of alloys: Brass – copper and ZINC n BRONZE – copper and tin p Sterling silver – SILVER and copper p
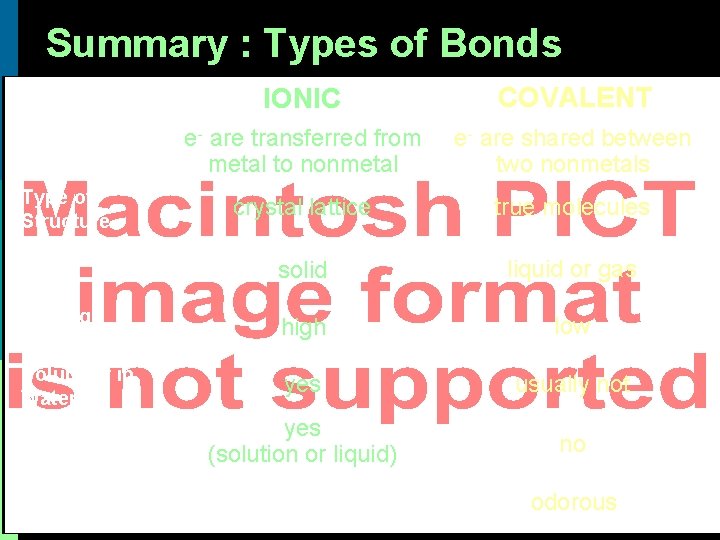
Summary : Types of Bonds IONIC COVALENT Bond Formation e- are transferred from metal to nonmetal e- are shared between two nonmetals Type of Structure crystal lattice true molecules Physical State solid liquid or gas Melting Point high low Solubility in Water yes usually not Electrical Conductivity yes (solution or liquid) no Other Properties odorous
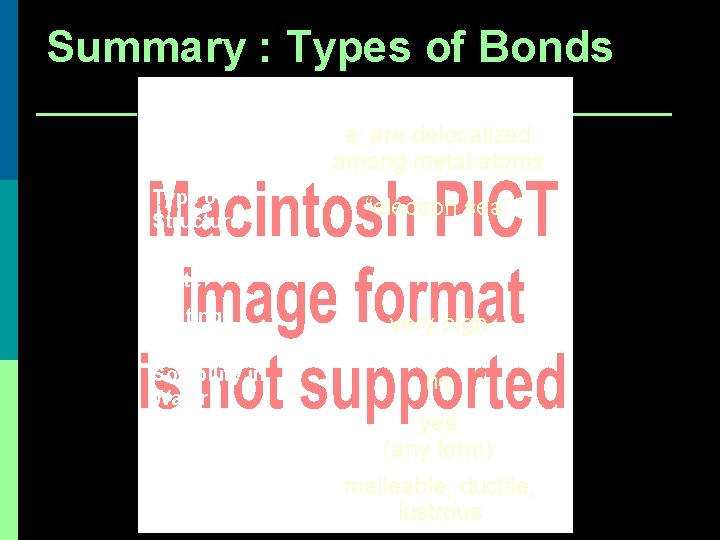
Summary : Types of Bonds METALLIC Bond Formation e- are delocalized among metal atoms Type of Structure “electron sea” Physical State solid Melting Point very high Solubility in Water no Electrical Conductivity yes (any form) Other Properties malleable, ductile, lustrous
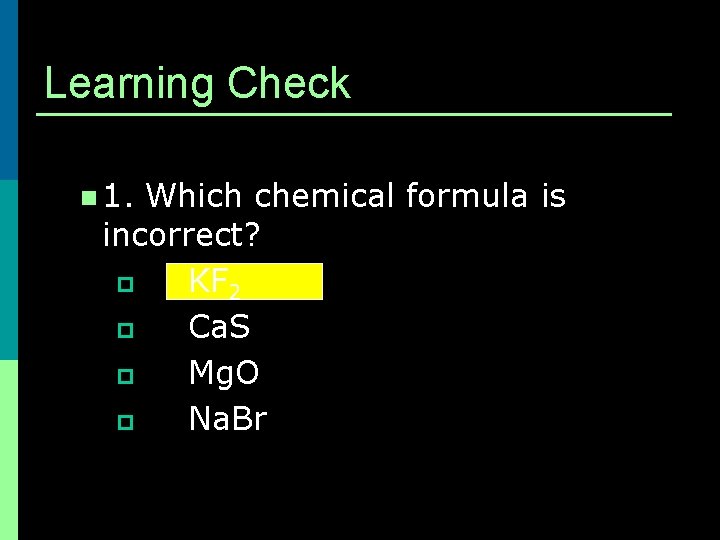
Learning Check n 1. Which chemical formula is incorrect? p KF 2 p Ca. S p Mg. O p Na. Br
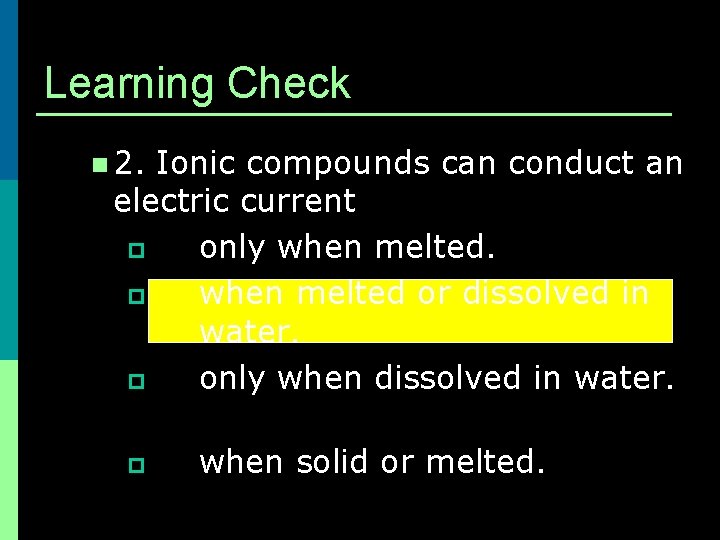
Learning Check n 2. Ionic compounds can conduct an electric current p only when melted. p when melted or dissolved in water. p only when dissolved in water. p when solid or melted.
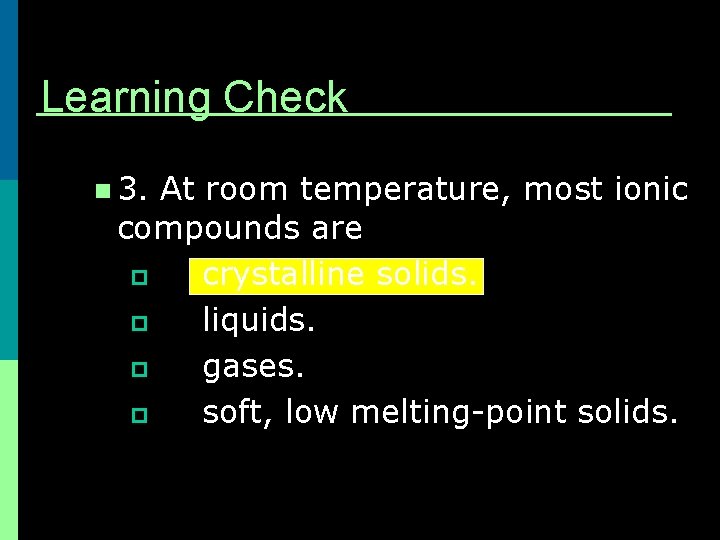
Learning Check n 3. At room temperature, most ionic compounds are p crystalline solids. p liquids. p gases. p soft, low melting-point solids.
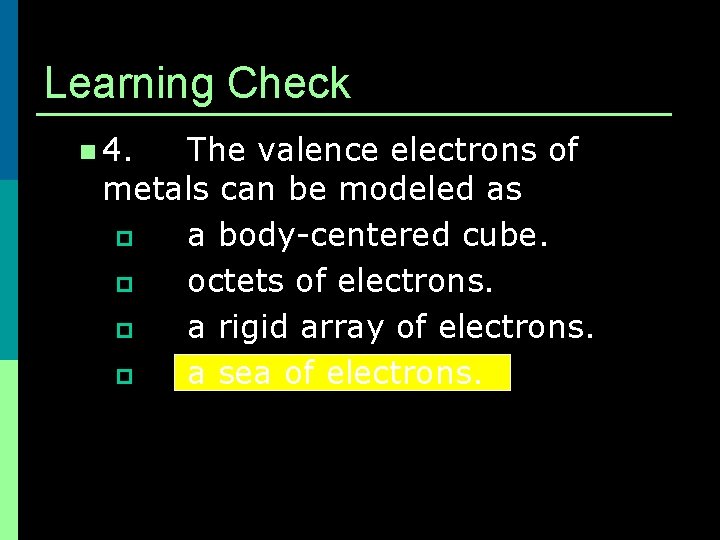
Learning Check n 4. The valence electrons of metals can be modeled as p a body-centered cube. p octets of electrons. p a rigid array of electrons. p a sea of electrons.

Learning Check n 5. In most metals, the atoms are pfree to move from one part of the metal to another. parranged in a compact and orderly pattern. pplaced at irregular locations. prandomly distributed.
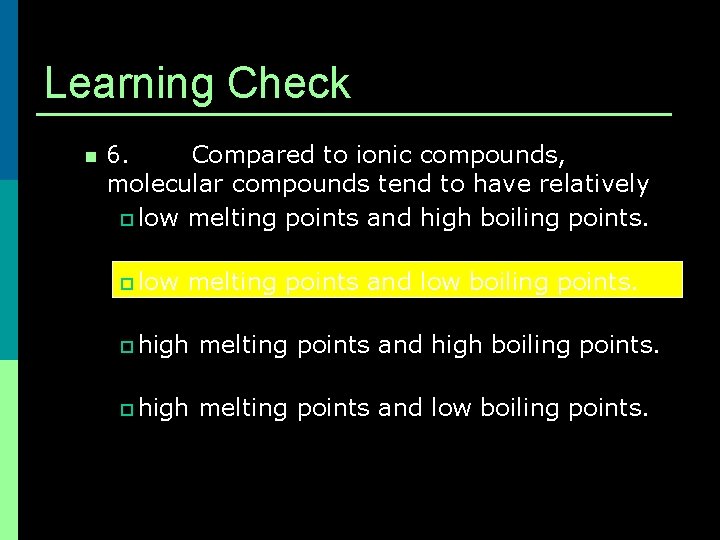
Learning Check n 6. Compared to ionic compounds, molecular compounds tend to have relatively p low melting points and high boiling points. p low melting points and low boiling points. p high melting points and high boiling points. p high melting points and low boiling points.

Learning Check n 7. A molecular compound usually consists of p two metal atoms and a nonmetal atom. p two nonmetal atoms and a metal atom. p two or more metal atoms. p two or more nonmetal atoms.

Assignment: Chose One Option p Option 1: STORY - Choose one type of bonding and write “A Day in the Life of an Atom” story describing what it's like to be an atom that forms your chosen bond type. The story should incorporate at least 5 properties from your Types of Bonds table.

Assignment: Chose One Option p Option 2: COMIC STRIP - Choose one type of bonding and write a comic strip with 3+ frames. The comic should incorporate at least 3 properties from your Types of Bonds table.

Assignment: Chose One Option p Option 3: SINGLE-FRAME CARTOON - Draw a single-frame cartoon for each type of bonding. Each cartoon should incorporate at least one key property from your Types of Bonds table.
- Slides: 30