Chemical Bonding Ionic Covalent Bonds Compounds A compound

Chemical Bonding Ionic & Covalent Bonds

Compounds �A compound is a substance whose smallest unit is made up of atoms of more than one element bonded together.

Compounds �Compounds often have properties that are different from the elements that make them up. � For Example: Water (H 2 O) and Hydrogen peroxide (H 2 O 2)
Chemical Bonding • The combining of atoms of elements to form new substances is called chemical bonding. electrons in the outermost shell are responsible for bonding. • Exposed
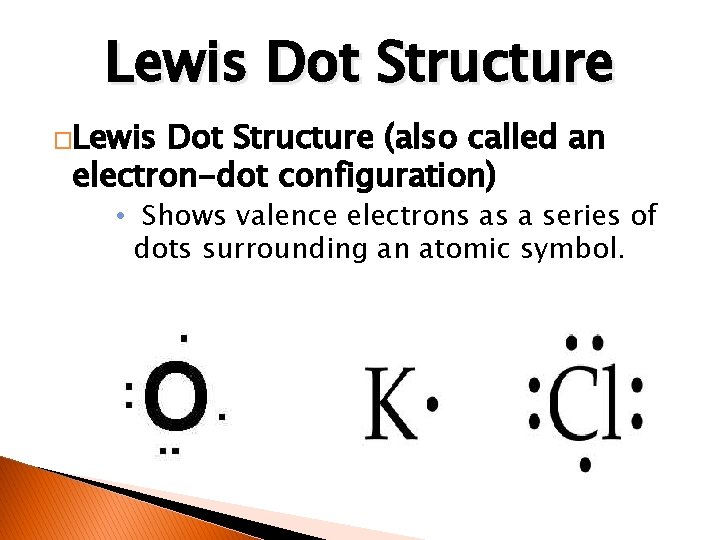
Lewis Dot Structure �Lewis Dot Structure (also called an electron-dot configuration) • Shows valence electrons as a series of dots surrounding an atomic symbol.
Lewis Dot Structure • Find out which group (column) your element is in. • This will tell you the number of valence electrons your element has. • You will only draw the valence electrons.
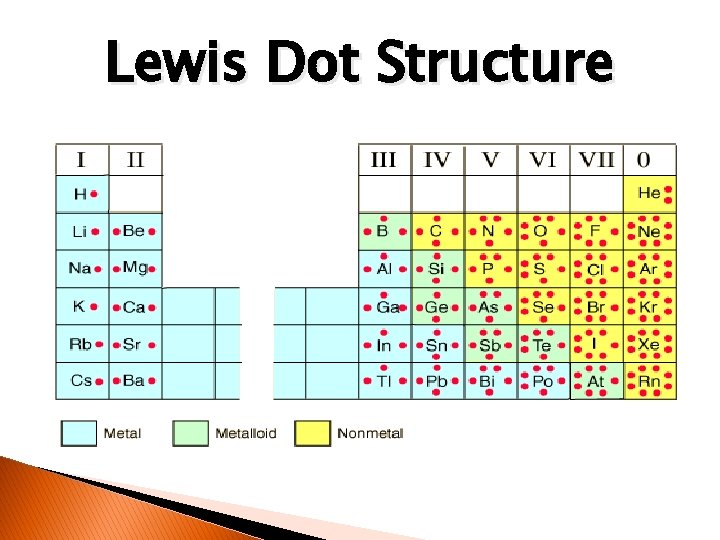
Lewis Dot Structure
The Octet Rule �Atoms will combine to form compounds in order to reach eight electrons in their outer energy level. � Be aware that there are some exceptions! q. CONSIDER EIGHT A HAPPY NUMBER FOR ATOMS!
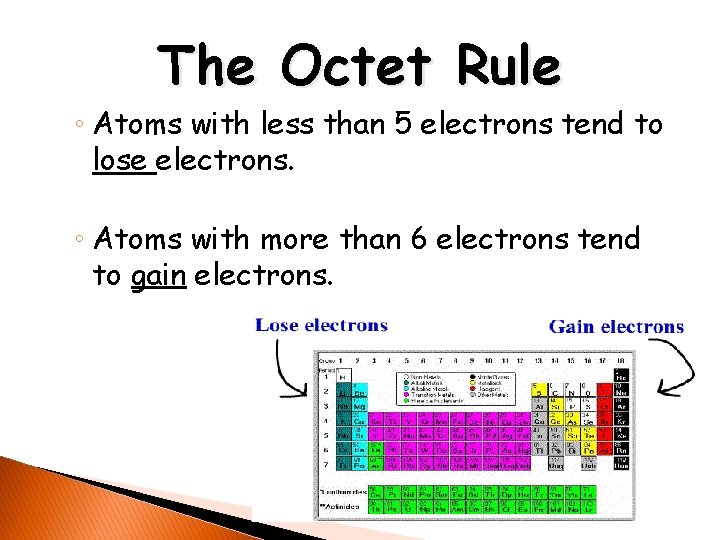
The Octet Rule ◦ Atoms with less than 5 electrons tend to lose electrons. ◦ Atoms with more than 6 electrons tend to gain electrons.
Ionic Bonds � � In ionic bonding, electrons are transferred between two or more atoms. Ion- is an atom having a positive or negative charge.

Ions atom that loses electrons becomes a positive ion. �The �Has more protons than electrons.

Ions atom that gains electrons becomes a negative ion. �The �Has more electrons than protons.
Ionic Bonds �The force of attraction between these oppositely charged ions is an IONIC BOND. �Ionic bonding occurs between a metal and a nonmetal.
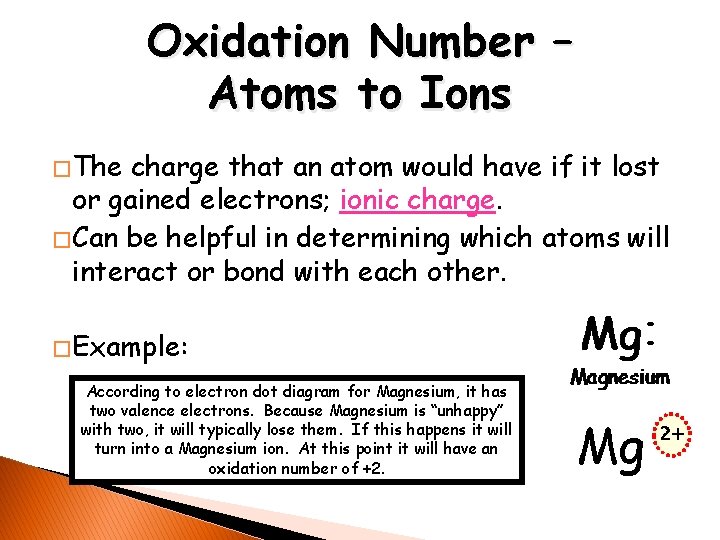
Oxidation Number – Atoms to Ions � The charge that an atom would have if it lost or gained electrons; ionic charge. � Can be helpful in determining which atoms will interact or bond with each other. � Example: According to electron dot diagram for Magnesium, it has two valence electrons. Because Magnesium is “unhappy” with two, it will typically lose them. If this happens it will turn into a Magnesium ion. At this point it will have an oxidation number of +2. Mg 2+
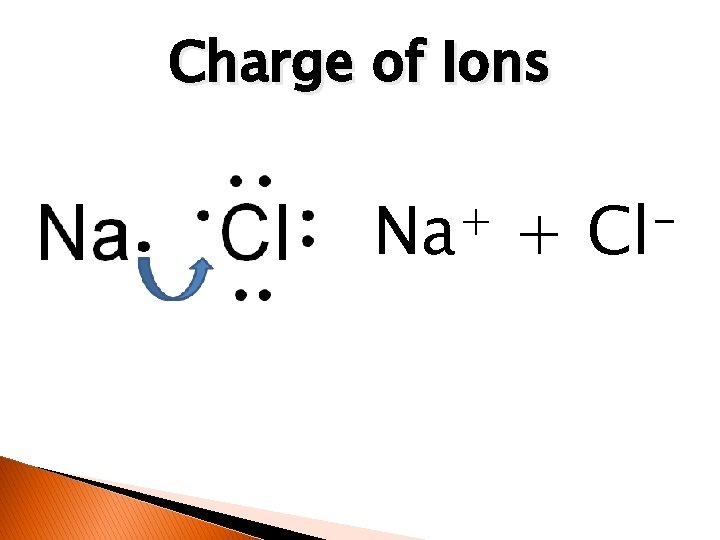
Charge of Ions + Na + Cl
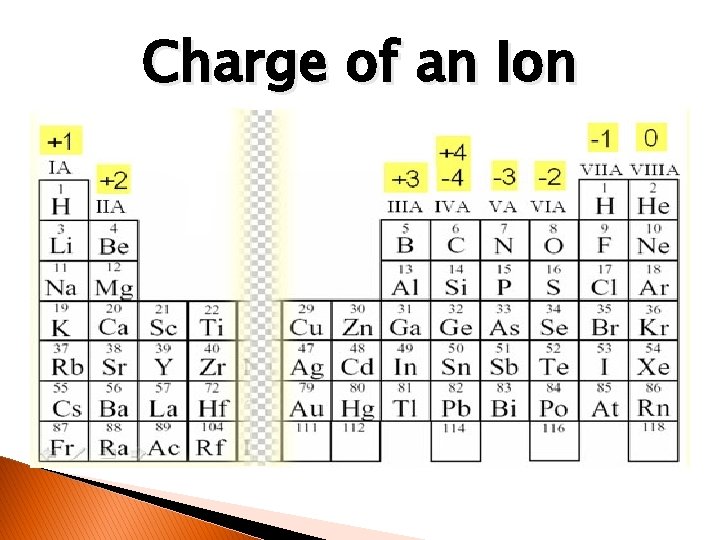
Charge of an Ion

�In Covalent Bonds covalent bonding, electrons are shared by two or more atoms. �Covalent bonding usually occurs between atoms of nonmetals.
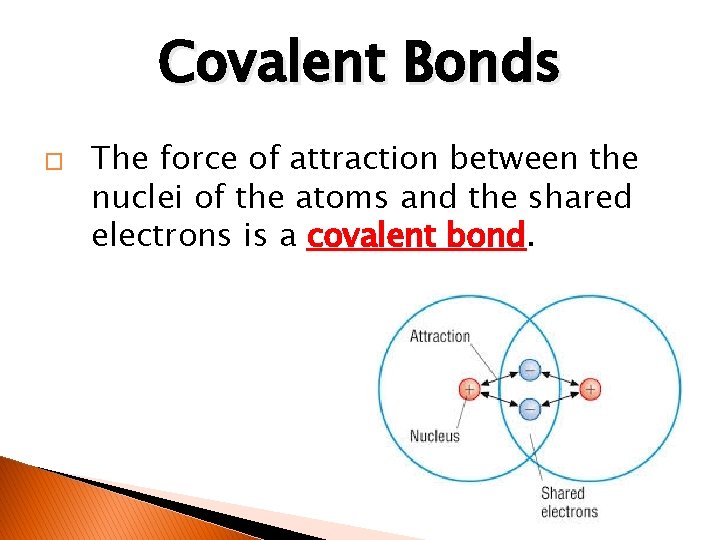
Covalent Bonds � The force of attraction between the nuclei of the atoms and the shared electrons is a covalent bond.
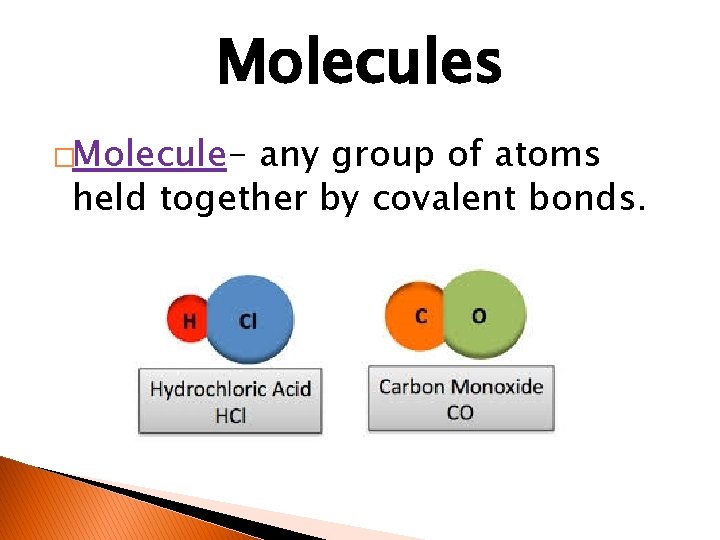
Molecules �Molecule- any group of atoms held together by covalent bonds.
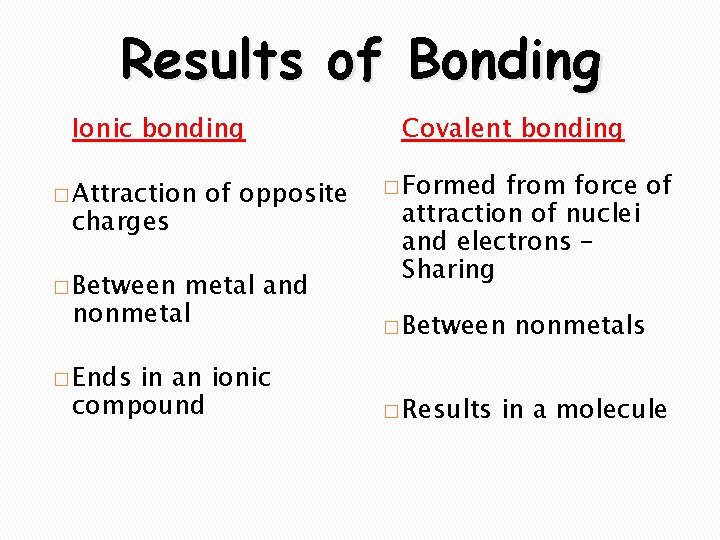
Results of Bonding Ionic bonding � Attraction charges of opposite � Between metal and nonmetal � Ends in an ionic compound Covalent bonding � Formed from force of attraction of nuclei and electrons – Sharing � Between � Results nonmetals in a molecule
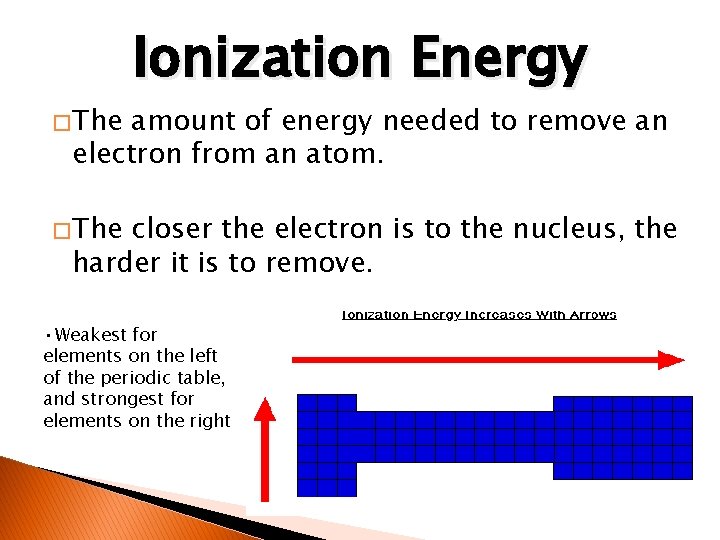
� The Ionization Energy amount of energy needed to remove an electron from an atom. � The closer the electron is to the nucleus, the harder it is to remove. • Weakest for elements on the left of the periodic table, and strongest for elements on the right
- Slides: 21