CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS

CHEMICAL BONDING • IONIC BONDS • COVALENT BONDS • HYDROGEN BONDS • METALLIC BONDS

IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence electrons

IONIC BONDING IS THE COMPOUND AN IONIC COMPOUND? METAL NONMETAL SUBSCRIPTS
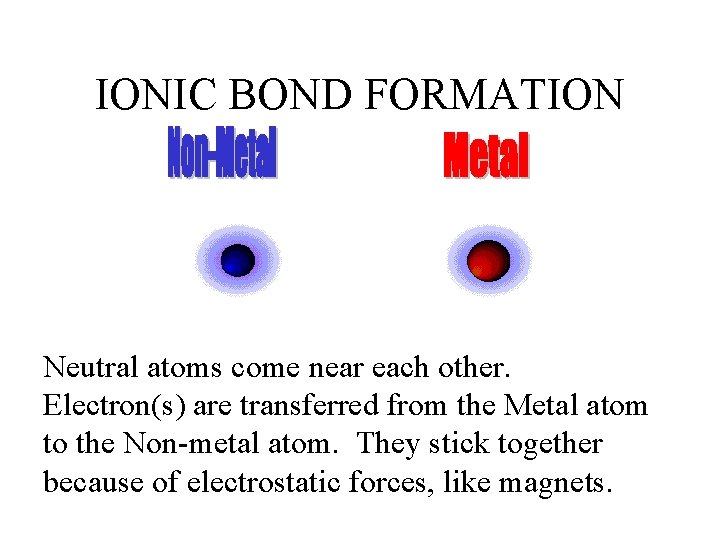
IONIC BOND FORMATION Neutral atoms come near each other. Electron(s) are transferred from the Metal atom to the Non-metal atom. They stick together because of electrostatic forces, like magnets.

IONIC BONDING Metals will tend to lose electrons and become POSITIVE CATIONS Normal sodium atom loses one electron to become sodium ion

IONIC BONDING Nonmetals will tend to gain electrons and become NEGATIVE ANIONS Normal chlorine atom gains an electron to become a chloride ion

IONIC BONDING POLYATOMIC IONS--a group of atoms that act like one ion +1 NH 4 --ammonium ion -2 CO 3 --carbonate ion -3 PO 4 --phosphate ion

IONIC BONDING SODIUM SULFATE

Properties of Ionic Compounds • Crystalline structure. • A regular repeating arrangement of ions in the solid. • Ions are strongly bonded. • Structure is rigid. • High melting points- because of strong forces between ions.
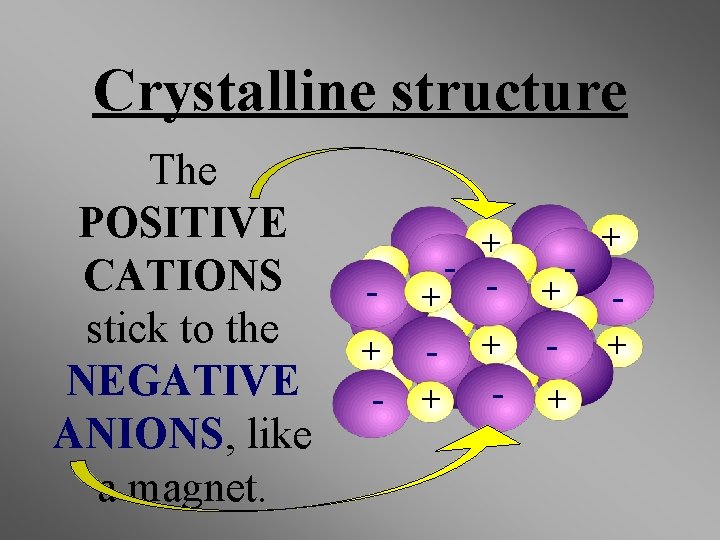
Crystalline structure The POSITIVE CATIONS stick to the NEGATIVE ANIONS, like a magnet. + - + - + + - - + +

Do they Conduct? • Conducting electricity is allowing charges to move. • In a solid, the ions are locked in place. • Ionic solids are insulators. • When melted, the ions can move around. • Melted ionic compounds conduct. • First get them to 800ºC. • Dissolved in water they conduct.
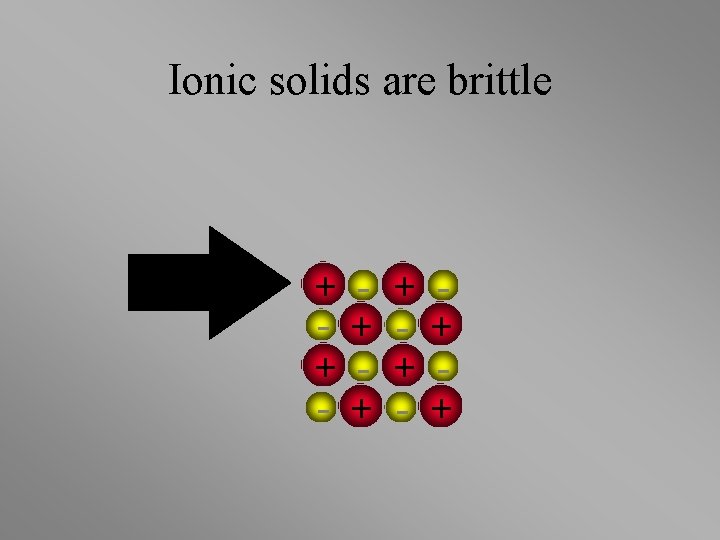
Ionic solids are brittle + + - + +
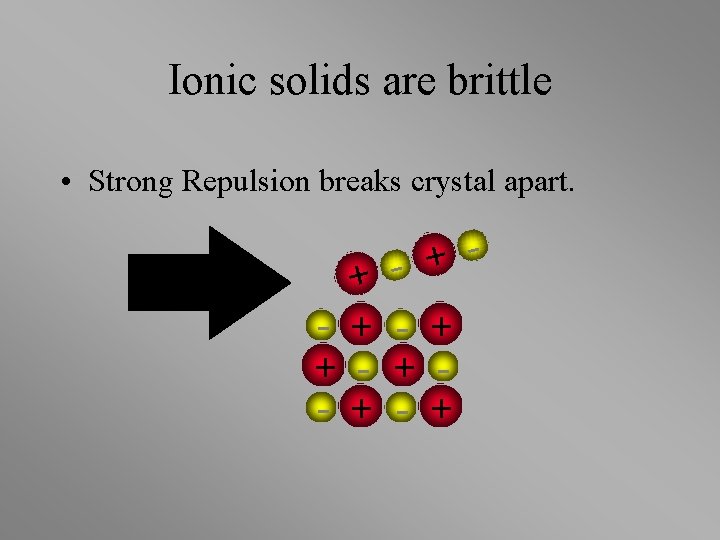
Ionic solids are brittle • Strong Repulsion breaks crystal apart. + + - + - + - +

COVALENT BONDING When an atom of one nonmetal shares one or more electrons with an atom of another nonmetal so both atoms end up with eight valence electrons
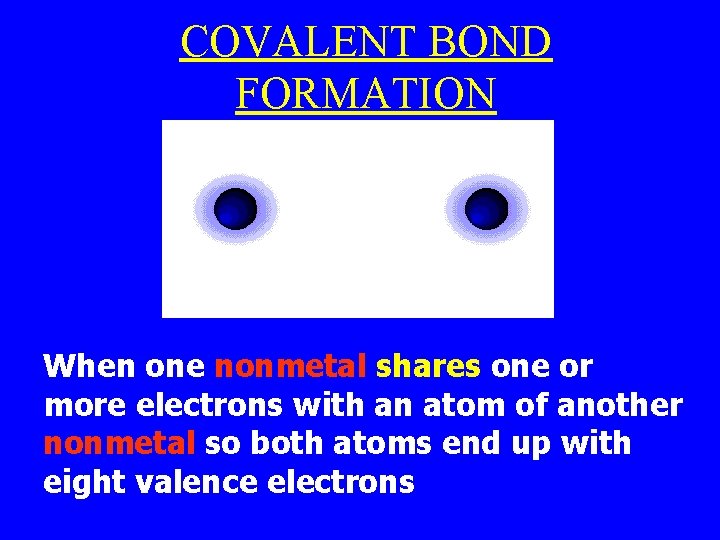
COVALENT BOND FORMATION When one nonmetal shares one or more electrons with an atom of another nonmetal so both atoms end up with eight valence electrons

COVALENT BONDING IS THE COMPOUND A COVALENT COMPOUND? NONMETAL YES since it is made of only nonmetal elements

Covalent bonding • Fluorine has seven valence electrons F

Covalent bonding • Fluorine has seven valence electrons • A second atom also has seven F F
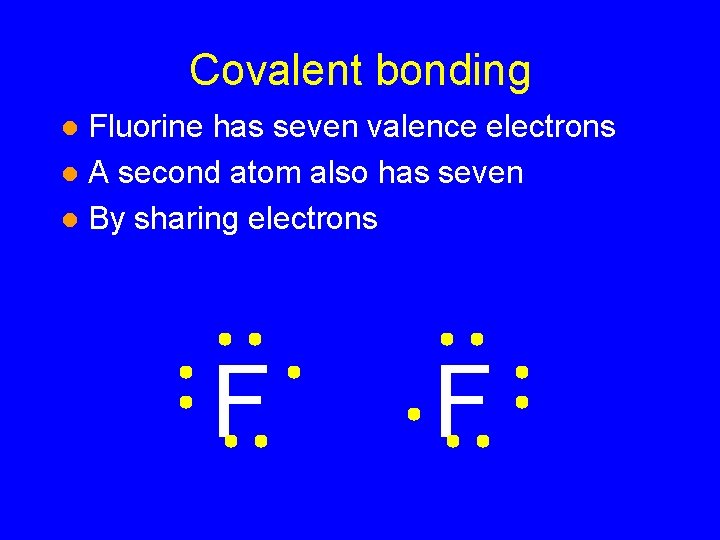
Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l F F
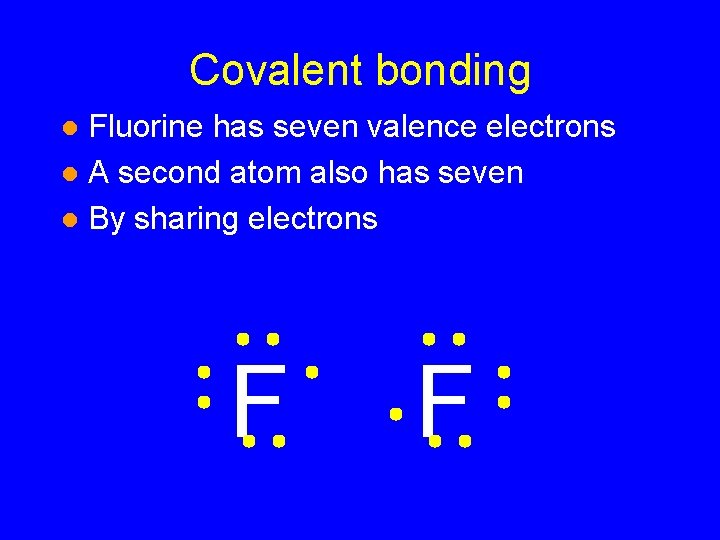
Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l F F

Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l F F

Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l F F

Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l F F

Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l Both end with full orbitals l F F
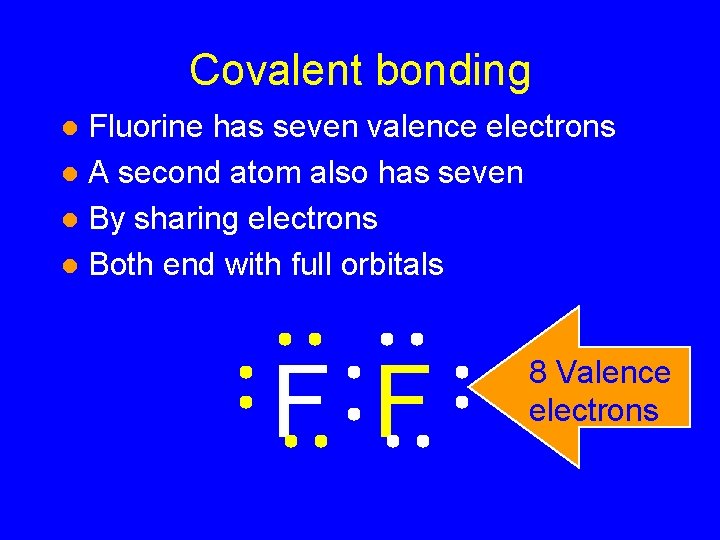
Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l Both end with full orbitals l F F 8 Valence electrons
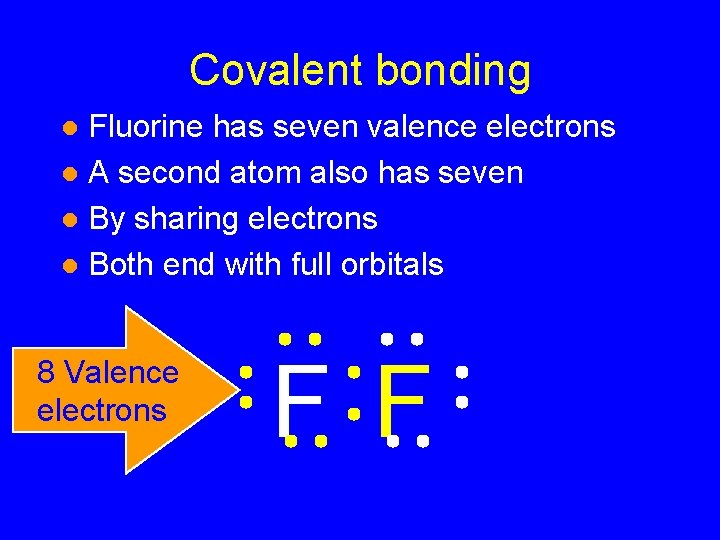
Covalent bonding Fluorine has seven valence electrons l A second atom also has seven l By sharing electrons l Both end with full orbitals l 8 Valence electrons F F

Single Covalent Bond • A sharing of two valence electrons. • Only nonmetals and Hydrogen. • Different from an ionic bond because they actually form molecules. • Two specific atoms are joined. • In an ionic solid you can’t tell which atom the electrons moved from or to.

Water H Each hydrogen has 1 valence electron Each hydrogen wants 1 more O The oxygen has 6 valence electrons The oxygen wants 2 more They share to make each other happy

Water • Put the pieces together • The first hydrogen is happy • The oxygen still wants one more HO
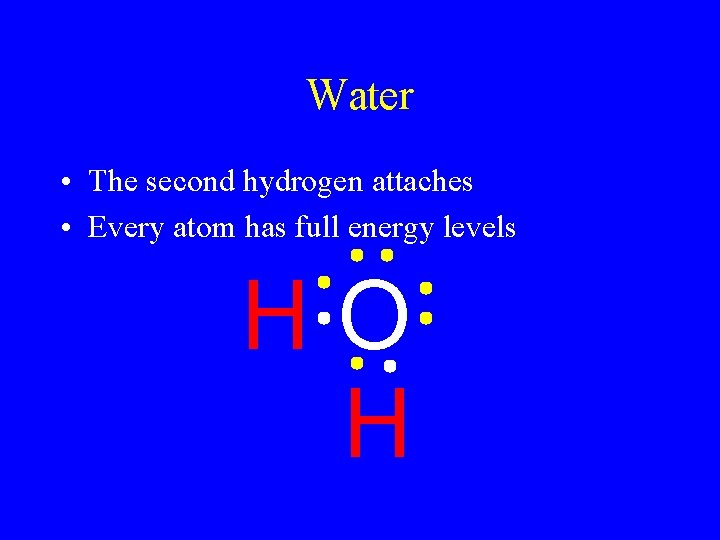
Water • The second hydrogen attaches • Every atom has full energy levels HO H

Carbon dioxide C O • CO 2 - Carbon is central atom ( I have to tell you) • Carbon has 4 valence electrons • Wants 4 more • Oxygen has 6 valence electrons • Wants 2 more

Carbon dioxide • Attaching 1 oxygen leaves the oxygen 1 short and the carbon 3 short CO
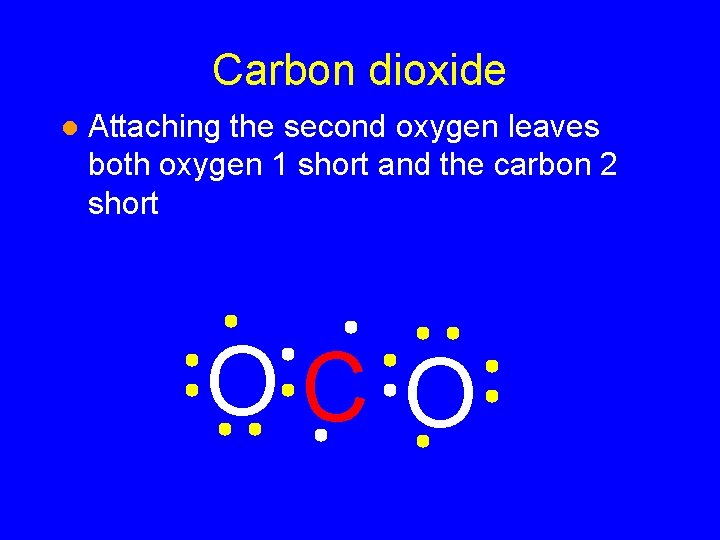
Carbon dioxide l Attaching the second oxygen leaves both oxygen 1 short and the carbon 2 short OC O
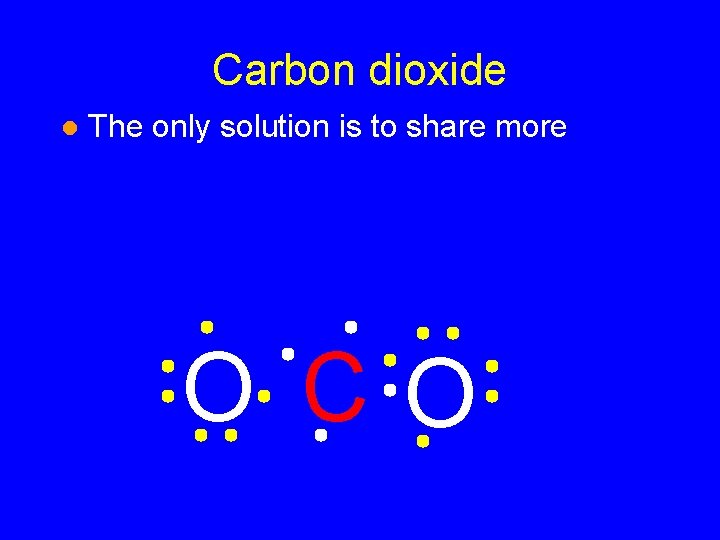
Carbon dioxide l The only solution is to share more O CO
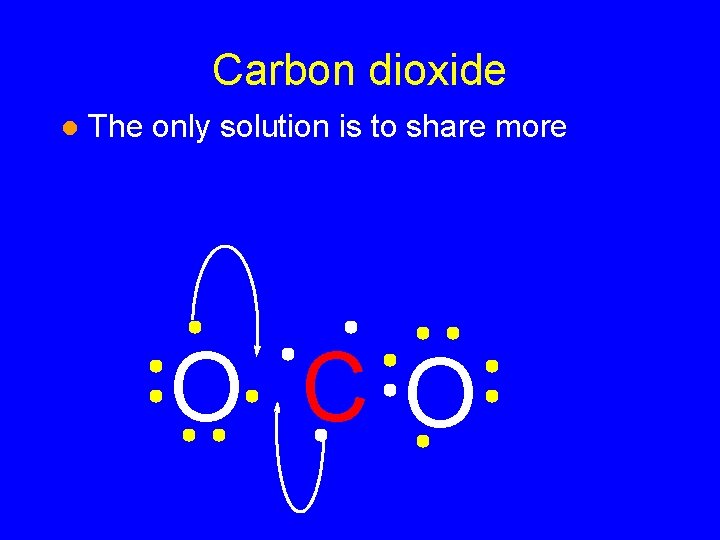
Carbon dioxide l The only solution is to share more O CO
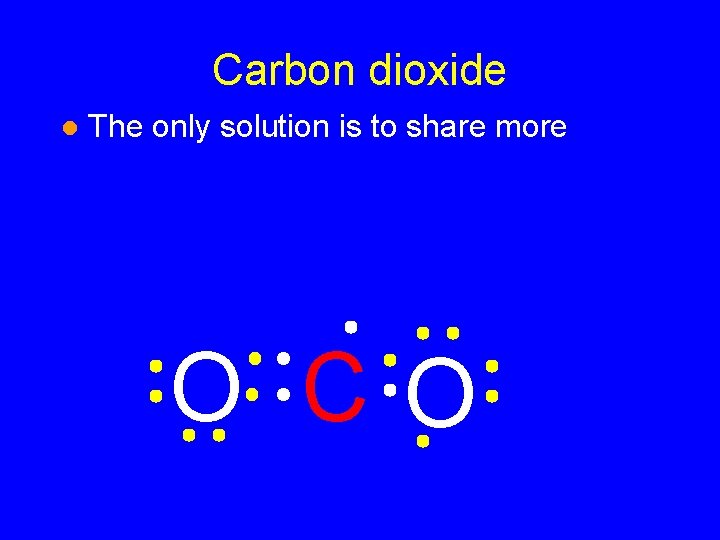
Carbon dioxide l The only solution is to share more O CO
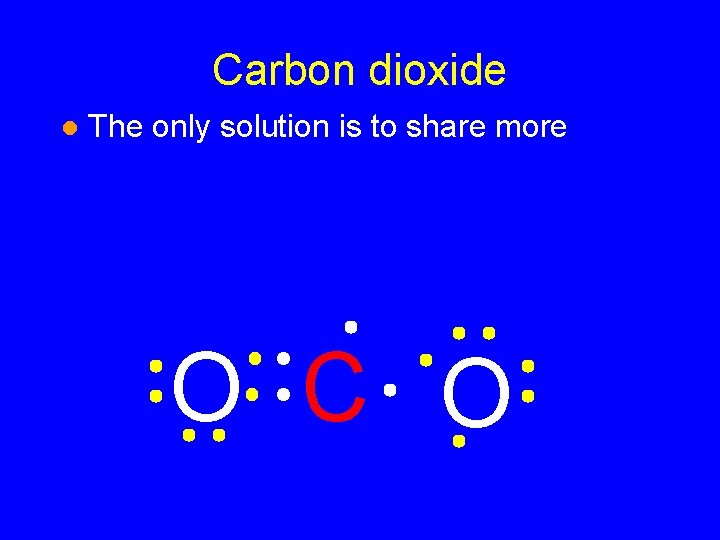
Carbon dioxide l The only solution is to share more O C O
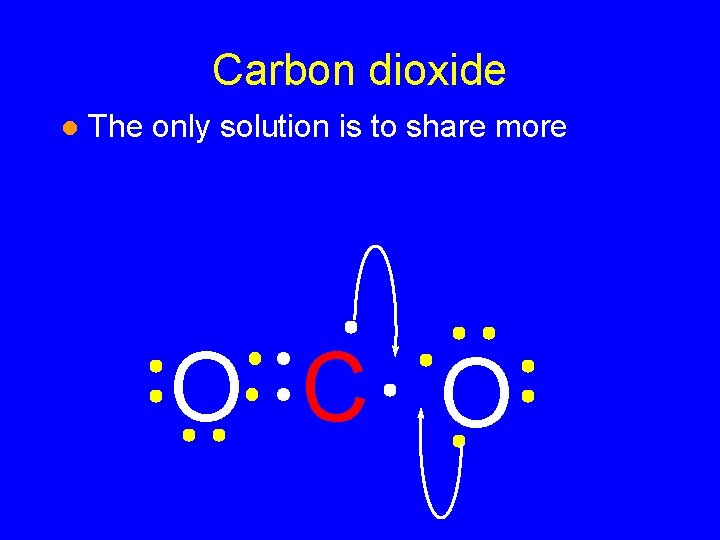
Carbon dioxide l The only solution is to share more O C O
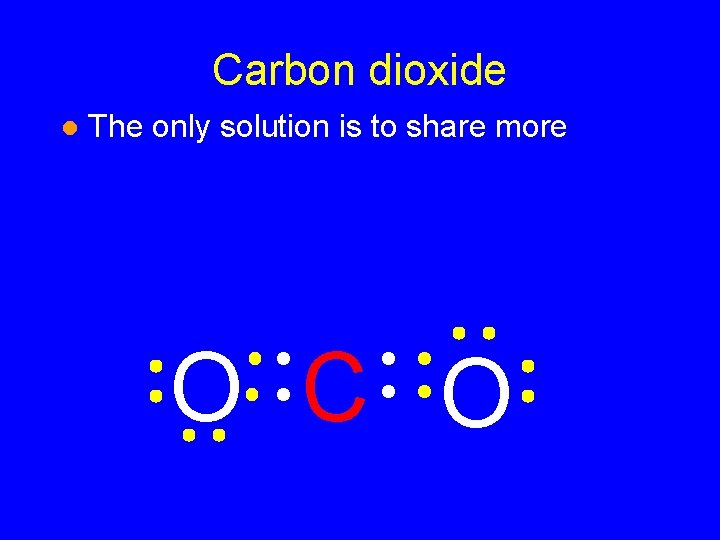
Carbon dioxide l The only solution is to share more O C O
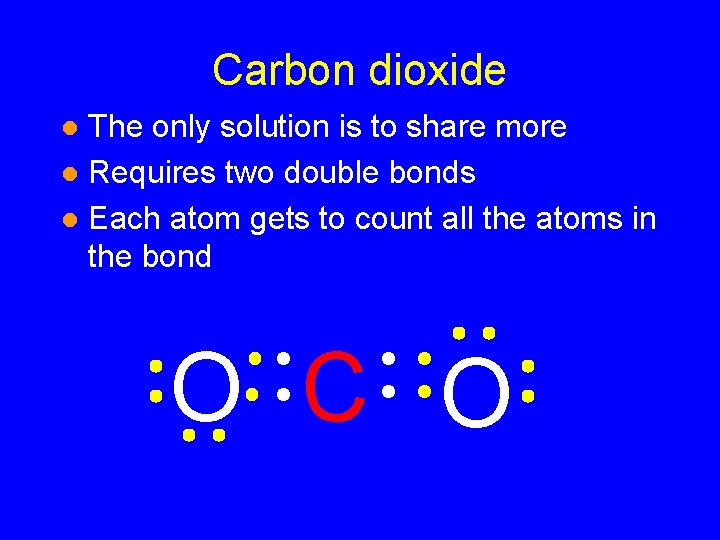
Carbon dioxide The only solution is to share more l Requires two double bonds l Each atom gets to count all the atoms in the bond l O C O
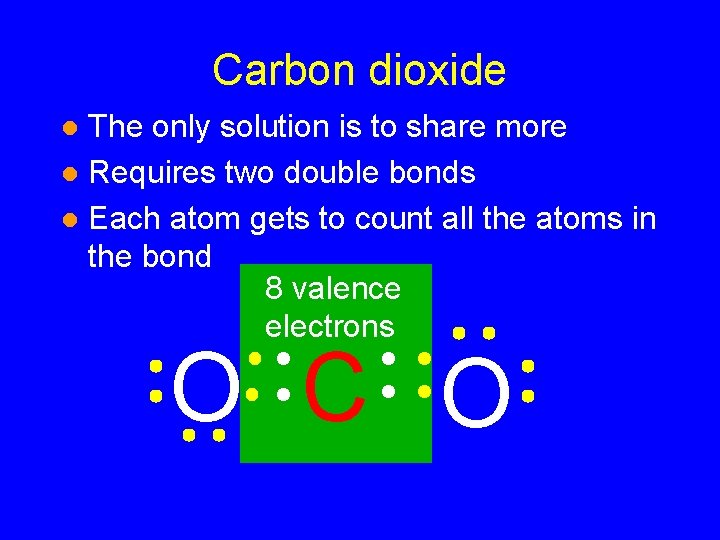
Carbon dioxide The only solution is to share more l Requires two double bonds l Each atom gets to count all the atoms in the bond 8 valence electrons l O C O
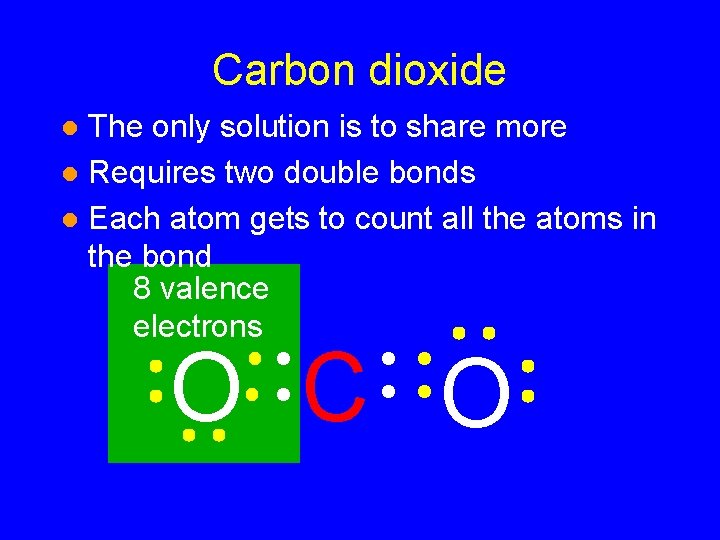
Carbon dioxide The only solution is to share more l Requires two double bonds l Each atom gets to count all the atoms in the bond 8 valence electrons l O C O
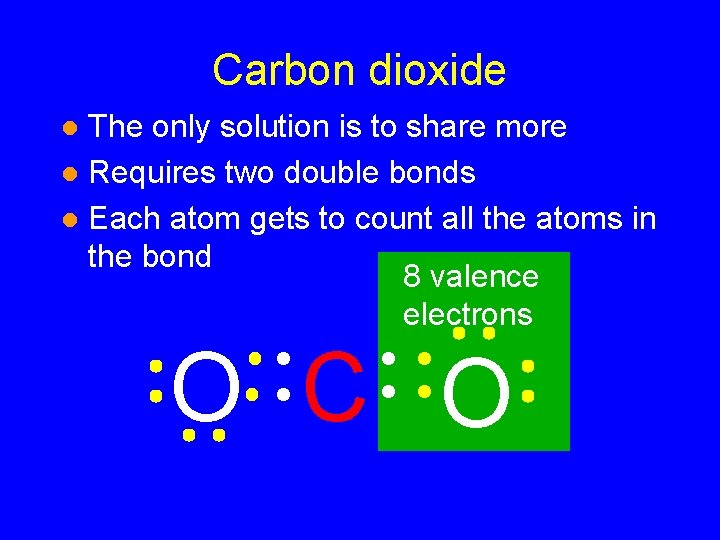
Carbon dioxide The only solution is to share more l Requires two double bonds l Each atom gets to count all the atoms in the bond 8 valence electrons l O C O

How to draw them • Add up all the valence electrons. • Count up the total number of electrons to make all atoms happy. • Subtract. • Divide by 2 • Tells you how many bonds - draw them. • Fill in the rest of the valence electrons to fill atoms up.

Examples • • HCN C is central atom N - has 5 valence electrons wants 8 C - has 4 valence electrons wants 8 H - has 1 valence electrons wants 2 HCN has 5+4+1 = 10 HCN wants 8+8+2 = 18 (18 -10)/2= 4 bonds 3 atoms with 4 bonds -will require multiple bonds - not to H

HCN • Put in single bonds • Need 2 more bonds • Must go between C and N HC N

HCN Put in single bonds l Need 2 more bonds l Must go between C and N l Uses 8 electrons - 2 more to add l HC N

HCN Put in single bonds l Need 2 more bonds l Must go between C and N l Uses 8 electrons - 2 more to add l Must go on N to fill octet l HC N

Polar Bonds • When the atoms in a bond are the same, the electrons are shared equally. • This is a nonpolar covalent bond. • When two different atoms are connected, the atoms may not be shared equally. • This is a polar covalent bond. • How do we measure how strong the atoms pull on electrons?

Electronegativity • A measure of how strongly the atoms attract electrons in a bond. • The bigger the electronegativity difference the more polar the bond. • 0. 0 - 0. 3 Covalent nonpolar • 0. 3 - 1. 67 Covalent polar • >1. 67 Ionic
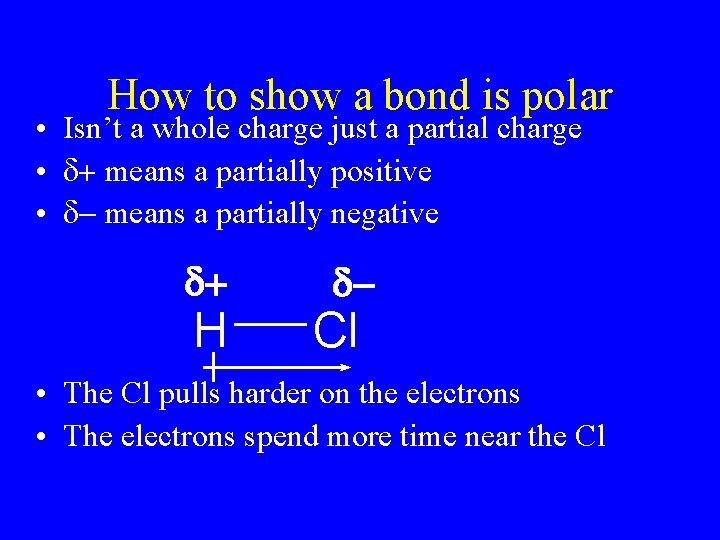
How to show a bond is polar • Isn’t a whole charge just a partial charge • d+ means a partially positive • d- means a partially negative d+ H d- Cl • The Cl pulls harder on the electrons • The electrons spend more time near the Cl

Polar Molecules with ends
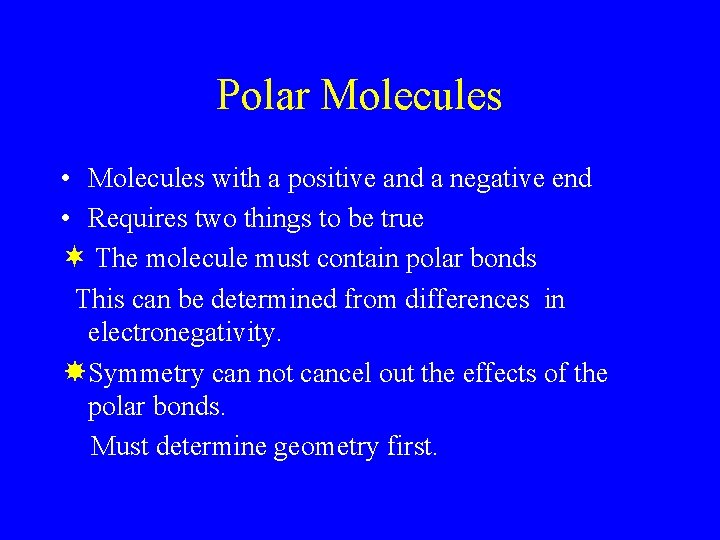
Polar Molecules • Molecules with a positive and a negative end • Requires two things to be true ¬ The molecule must contain polar bonds This can be determined from differences in electronegativity. Symmetry can not cancel out the effects of the polar bonds. Must determine geometry first.

Is it polar? • HF • H 2 O • NH 3 • CCl 4 • CO 2
Intermolecular Forces What holds molecules to each other

Intermolecular Forces • They are what make solid and liquid molecular compounds possible. • The weakest are called van der Waal’s forces there are two kinds • Dispersion forces • Dipole Interactions – depend on the number of electrons – more electrons stronger forces – Bigger molecules
Dipole interactions • Depend on the number of electrons • More electrons stronger forces • Bigger molecules more electrons • Fluorine is a gas • Bromine is a liquid • Iodine is a solid

Dipole interactions • Occur when polar molecules are attracted to each other. • Slightly stronger than dispersion forces. • Opposites attract but not completely hooked like in ionic solids.
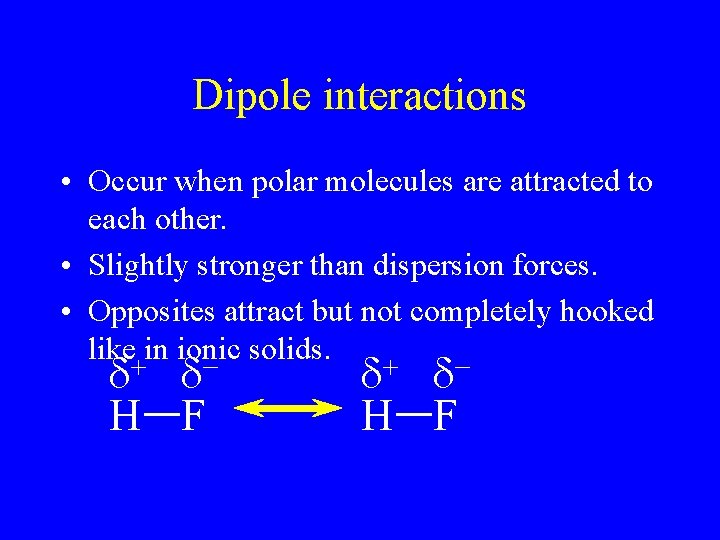
Dipole interactions • Occur when polar molecules are attracted to each other. • Slightly stronger than dispersion forces. • Opposites attract but not completely hooked like+in ionic solids. + - d d H F
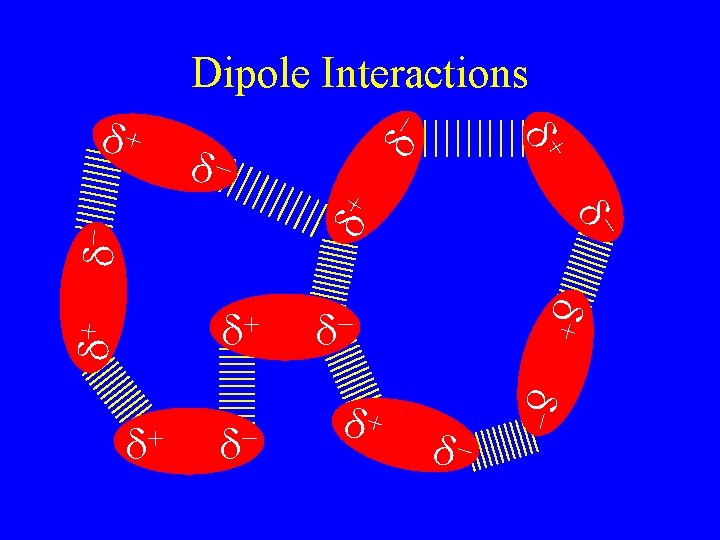
dd+ - d- d d+ d- d- d+ d+ + d d d+ + d- d d+ d- Dipole Interactions

Hydrogen bonding • Are the attractive force caused by hydrogen bonded to F, O, or N. • F, O, and N are very electronegative so it is a very strong dipole. • The hydrogen partially share with the lone pair in the molecule next to it. • The strongest of the intermolecular forces.
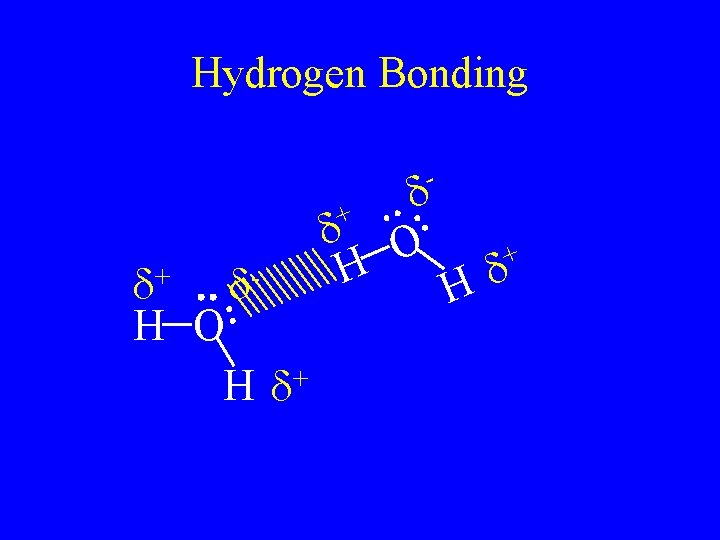
Hydrogen Bonding - + d+ d. H O + Hd d H d O + d H
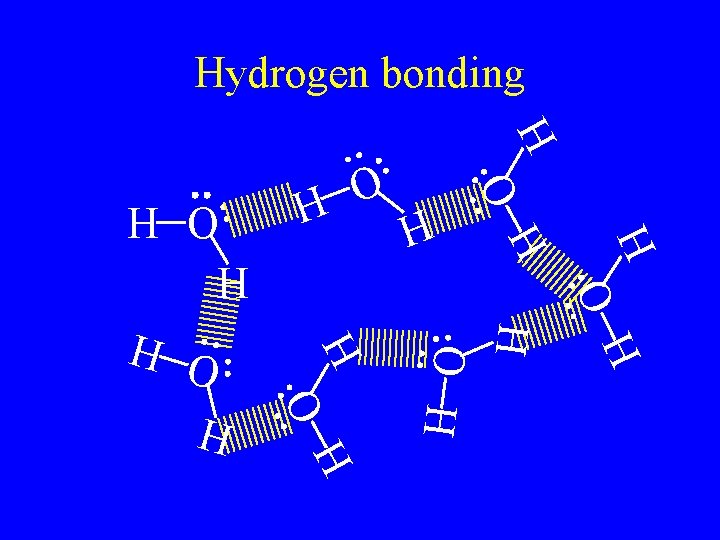
H H O H H O O H Hydrogen bonding
MOLECULAR SHAPES OF COVALENT COMPOUNDS

VSep. R t. HEORY
What Vsepr means Since electrons do not like each other, because of their negative charges, they orient themselves as far apart as possible, from each other. This leads to molecules having specific shapes.
Things to remember • Atoms bond to form an Octet (8 outer electrons/full outer energy level) • Bonded electrons take up less space then un-bonded/unshared pairs of electrons.
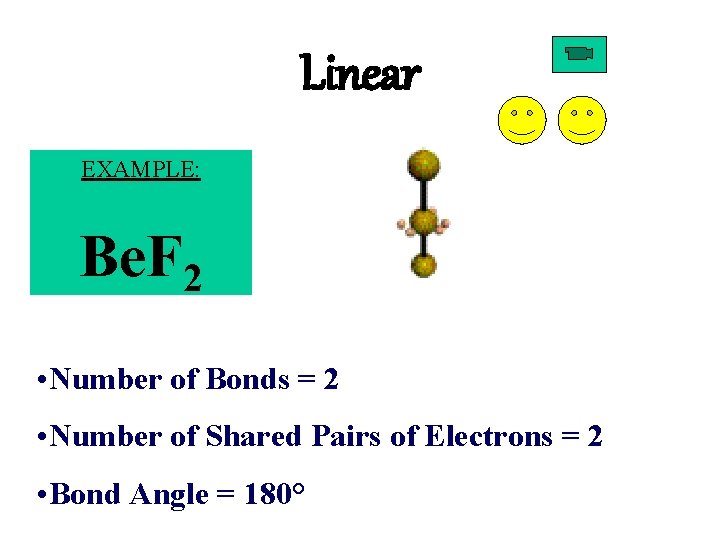
Linear EXAMPLE: Be. F 2 • Number of Bonds = 2 • Number of Shared Pairs of Electrons = 2 • Bond Angle = 180°
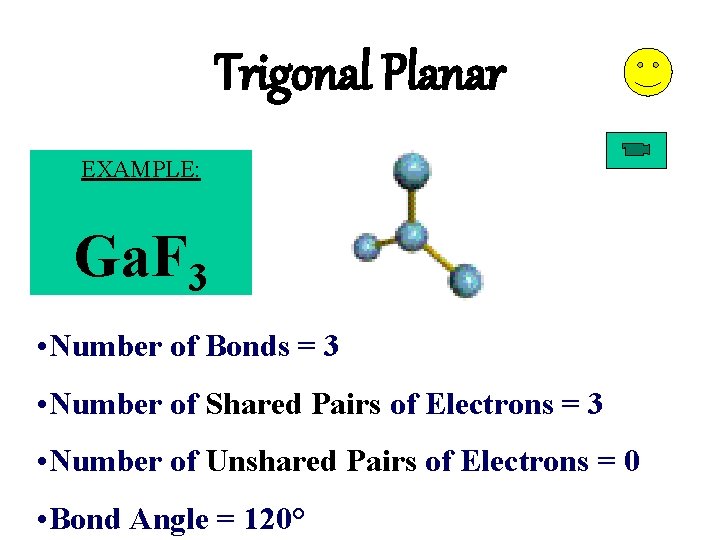
Trigonal Planar EXAMPLE: Ga. F 3 • Number of Bonds = 3 • Number of Shared Pairs of Electrons = 3 • Number of Unshared Pairs of Electrons = 0 • Bond Angle = 120°
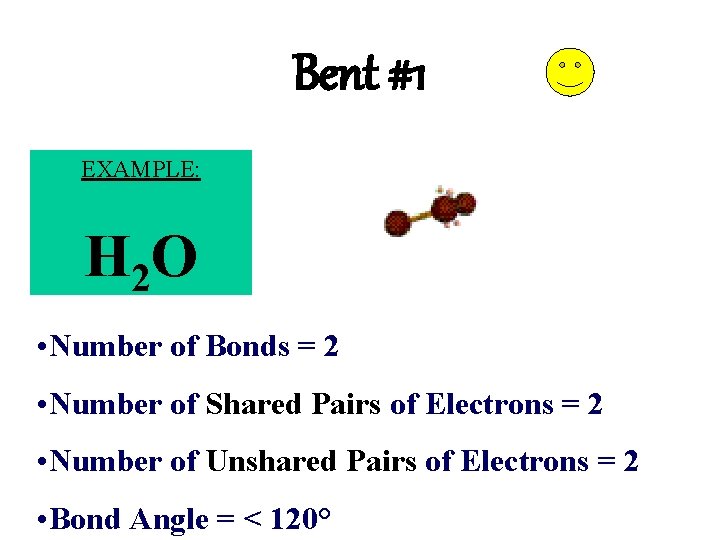
Bent #1 EXAMPLE: H 2 O • Number of Bonds = 2 • Number of Shared Pairs of Electrons = 2 • Number of Unshared Pairs of Electrons = 2 • Bond Angle = < 120°
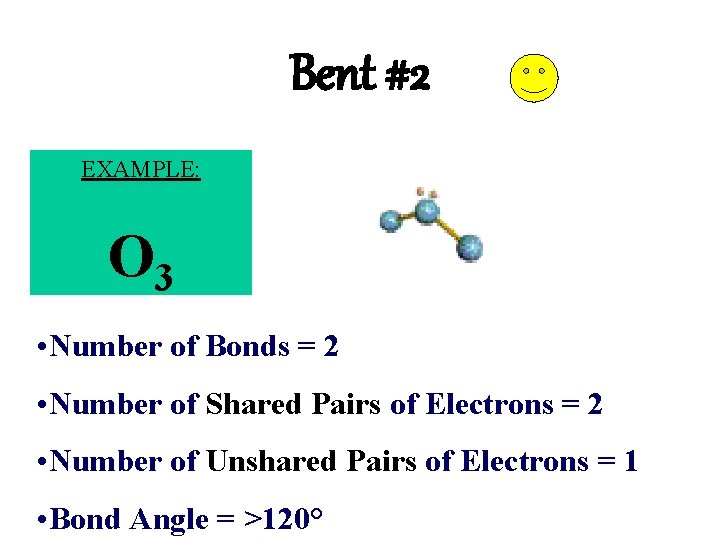
Bent #2 EXAMPLE: O 3 • Number of Bonds = 2 • Number of Shared Pairs of Electrons = 2 • Number of Unshared Pairs of Electrons = 1 • Bond Angle = >120°
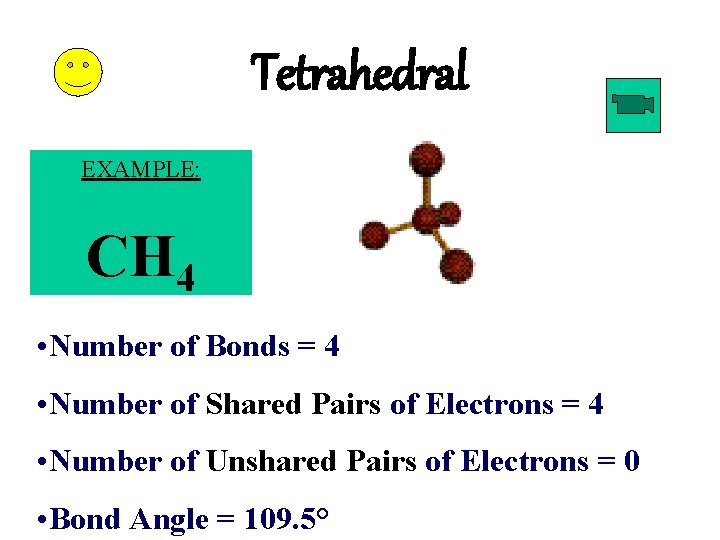
Tetrahedral EXAMPLE: CH 4 • Number of Bonds = 4 • Number of Shared Pairs of Electrons = 4 • Number of Unshared Pairs of Electrons = 0 • Bond Angle = 109. 5°
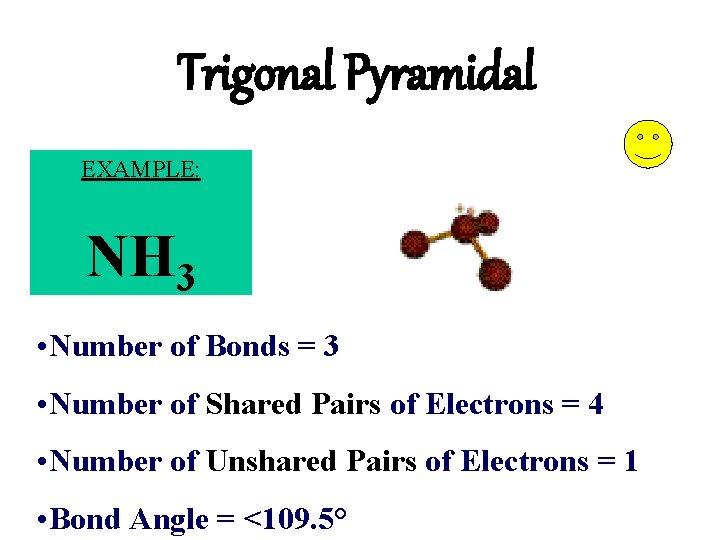
Trigonal Pyramidal EXAMPLE: NH 3 • Number of Bonds = 3 • Number of Shared Pairs of Electrons = 4 • Number of Unshared Pairs of Electrons = 1 • Bond Angle = <109. 5°
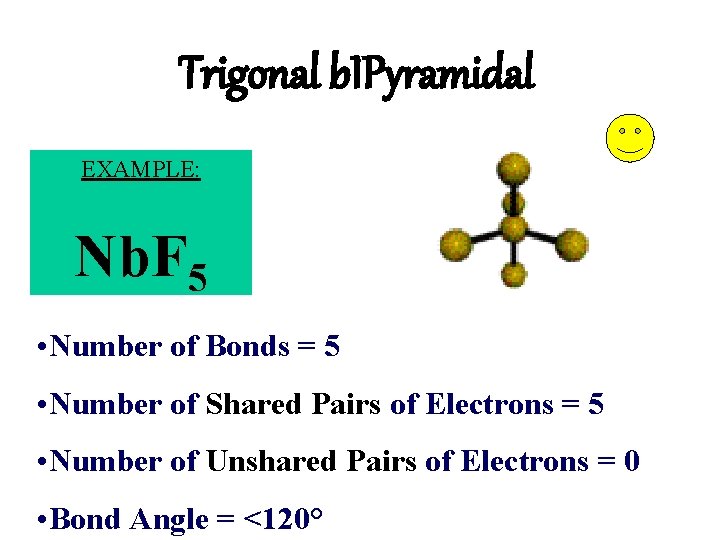
Trigonal b. IPyramidal EXAMPLE: Nb. F 5 • Number of Bonds = 5 • Number of Shared Pairs of Electrons = 5 • Number of Unshared Pairs of Electrons = 0 • Bond Angle = <120°
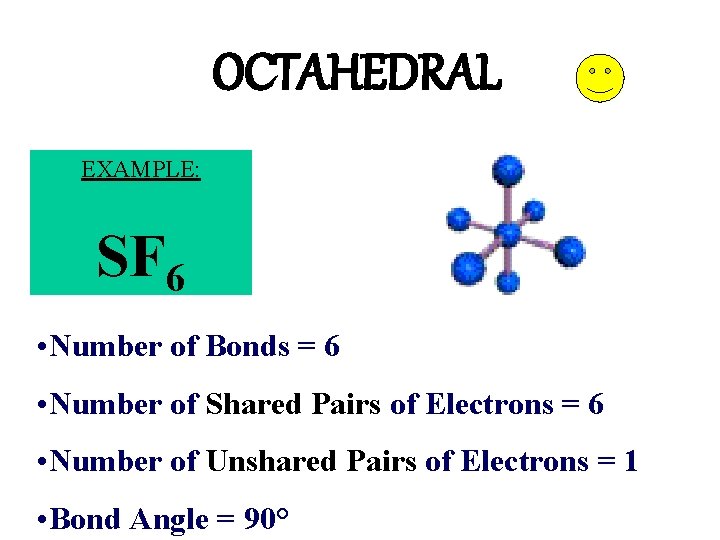
OCTAHEDRAL EXAMPLE: SF 6 • Number of Bonds = 6 • Number of Shared Pairs of Electrons = 6 • Number of Unshared Pairs of Electrons = 1 • Bond Angle = 90°

Metallic Bonds • How atoms are held together in the solid. • Metals hold onto there valence electrons very weakly. • Think of them as positive ions floating in a sea of electrons.
Sea of Electrons • Electrons are free to move through the solid. • Metals conduct electricity. + + +

Metals are Malleable • Hammered into shape (bend). • Ductile - drawn into wires.
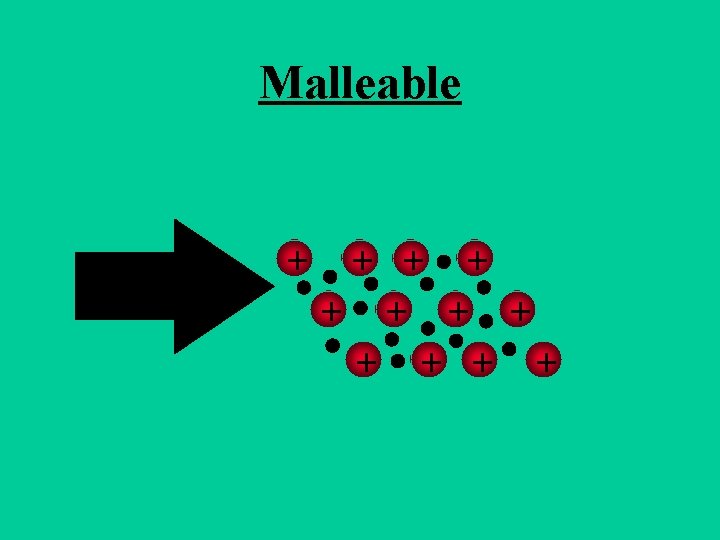
Malleable + + +
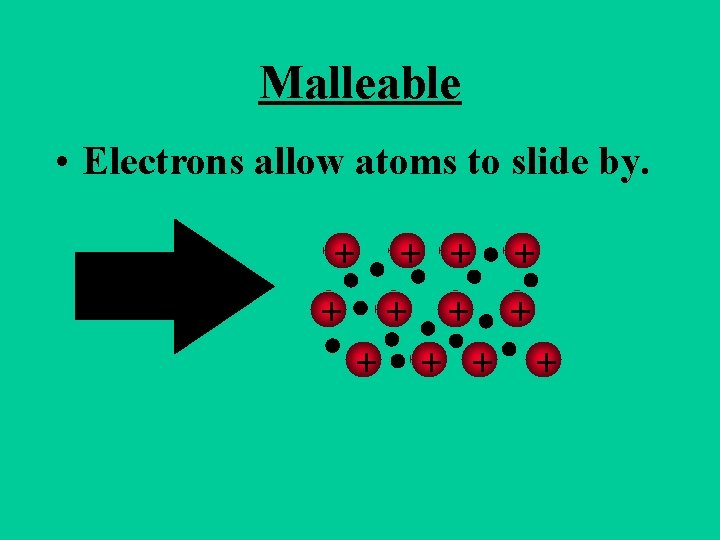
Malleable • Electrons allow atoms to slide by. + + +

- Slides: 84