Chemical Bonding Introduction to Chemical Bonding Introduction to

Chemical Bonding Introduction to Chemical Bonding
Introduction to Chemical Bonding Objectives Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding is neither purely ionic nor purely covalent Classify bonding type according to electronegativity differences

Introduction to Chemical Bonding
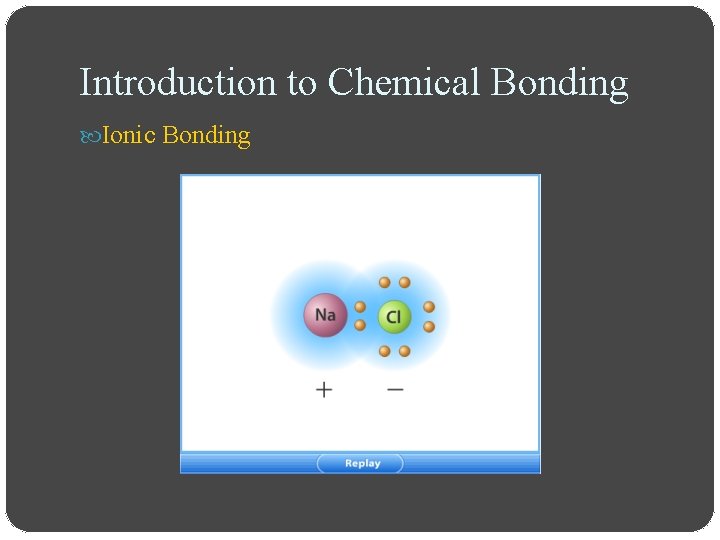
Introduction to Chemical Bonding Ionic Bonding
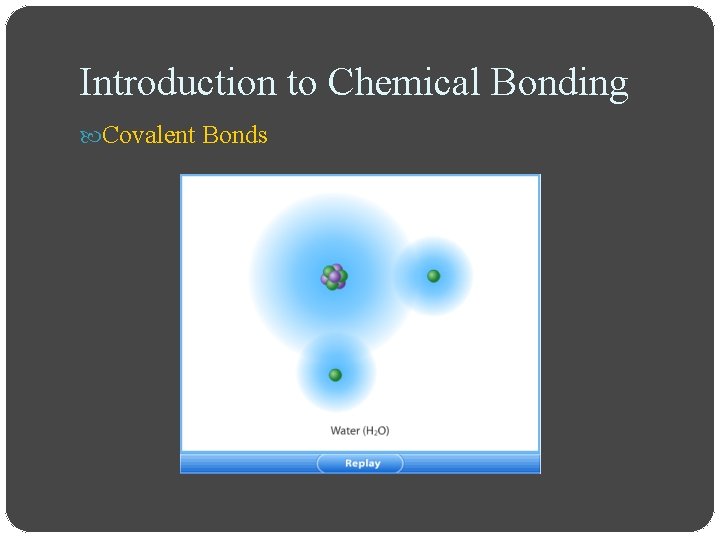
Introduction to Chemical Bonding Covalent Bonds
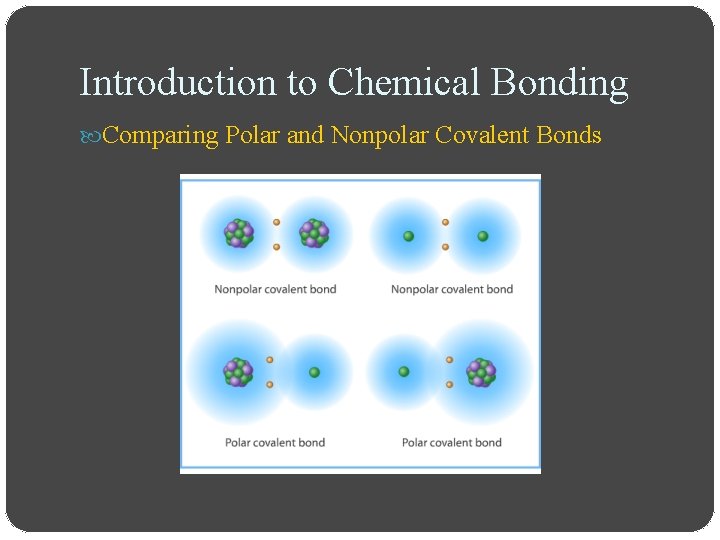
Introduction to Chemical Bonding Comparing Polar and Nonpolar Covalent Bonds
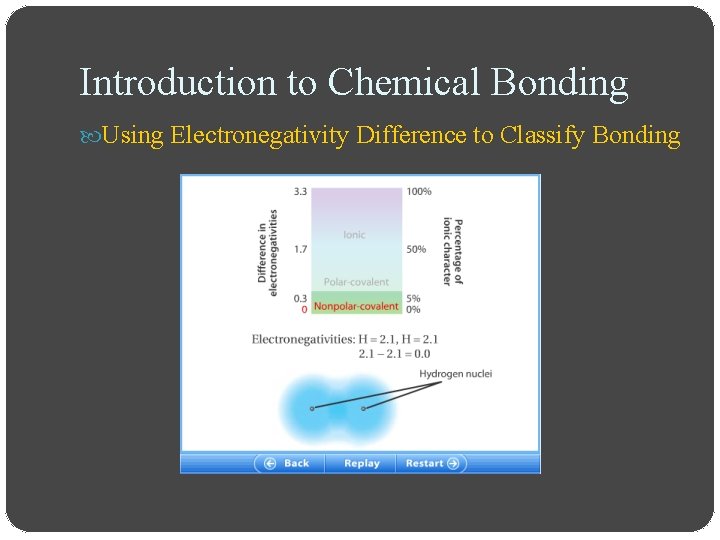
Introduction to Chemical Bonding Using Electronegativity Difference to Classify Bonding
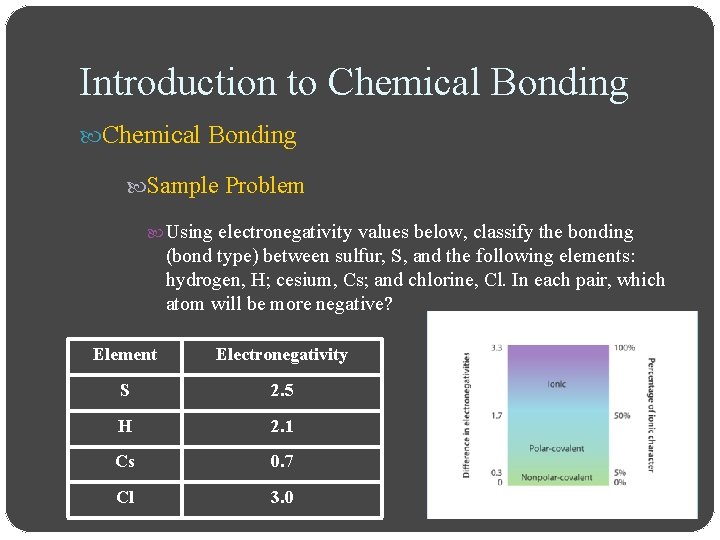
Introduction to Chemical Bonding Sample Problem Using electronegativity values below, classify the bonding (bond type) between sulfur, S, and the following elements: hydrogen, H; cesium, Cs; and chlorine, Cl. In each pair, which atom will be more negative? Element Electronegativity S 2. 5 H 2. 1 Cs 0. 7 Cl 3. 0
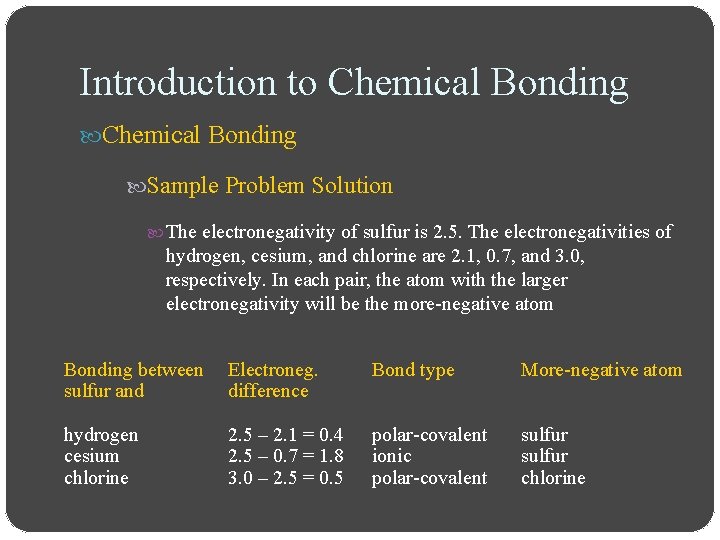
Introduction to Chemical Bonding Sample Problem Solution The electronegativity of sulfur is 2. 5. The electronegativities of hydrogen, cesium, and chlorine are 2. 1, 0. 7, and 3. 0, respectively. In each pair, the atom with the larger electronegativity will be the more-negative atom Bonding between sulfur and Electroneg. difference Bond type More-negative atom hydrogen cesium chlorine 2. 5 – 2. 1 = 0. 4 2. 5 – 0. 7 = 1. 8 3. 0 – 2. 5 = 0. 5 polar-covalent ionic polar-covalent sulfur chlorine
- Slides: 9