Chemical BONDING Intermolecular Attractions Intermolecular attractions Attractions between

Chemical BONDING Intermolecular Attractions
Intermolecular attractions • Attractions between molecules 1. London Dispersion Forces • Weak attractive forces between non-polar molecules – Ex. CH 4, CO 2 & Halogen molecules • Strongest LDF between molecules with LOTS of electrons.

Rank in order of weak to strong L-D • F 2, I 2, Br 2, Cl 2 • H 2, CH 4, CCl 4 • I 2, CF 4, CO 2
2. Dipole-dipole attractions • Stronger attractions that occur between polar molecules (still only 1% of the strength of a covalent bond) Hydrogen “Bonding” –Special type of dipole-dipole force found ONLY between FH - - F OH - - O NH - - N

Why does H “bonding” occur with only these 3 atoms? • Nitrogen, Oxygen and Fluorine – 3 highest electronegativities – smallest atoms with strongest nuclear charges
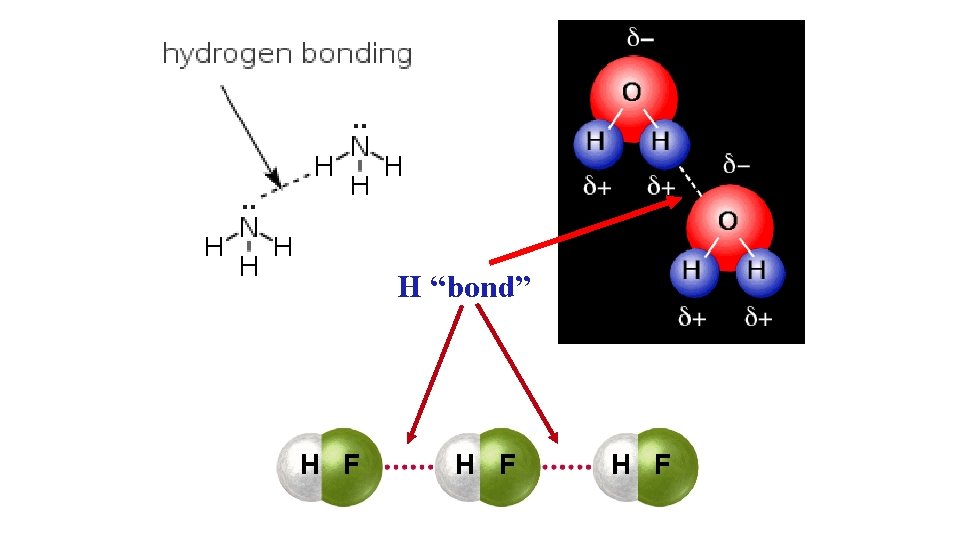
H “bond” H F H F
Intermolecular forces determine the properties (behavior) of the substance • Stronger IMFs, result in higher boiling points, higher melting points and slower evaporation of substances.
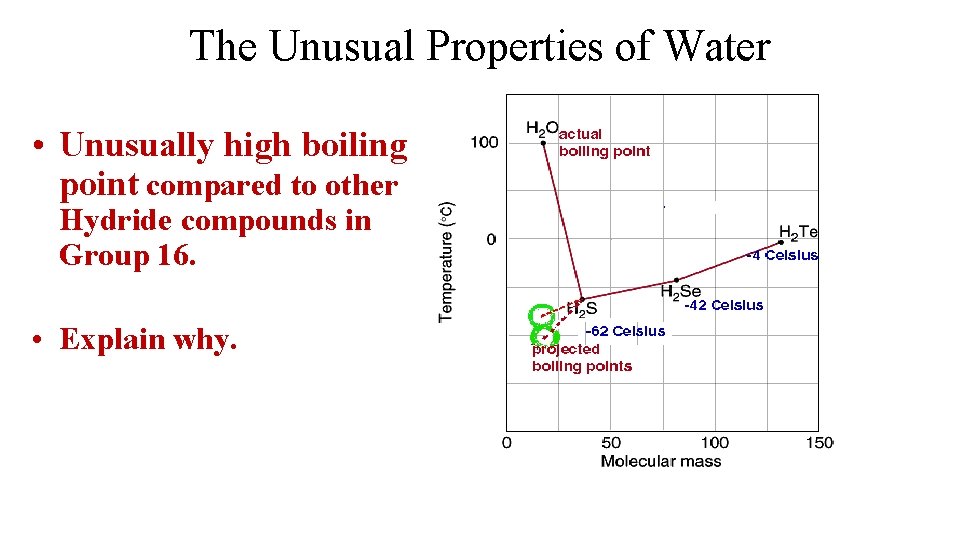
The Unusual Properties of Water • Unusually high boiling point compared to other Hydride compounds in Group 16. • Explain why.
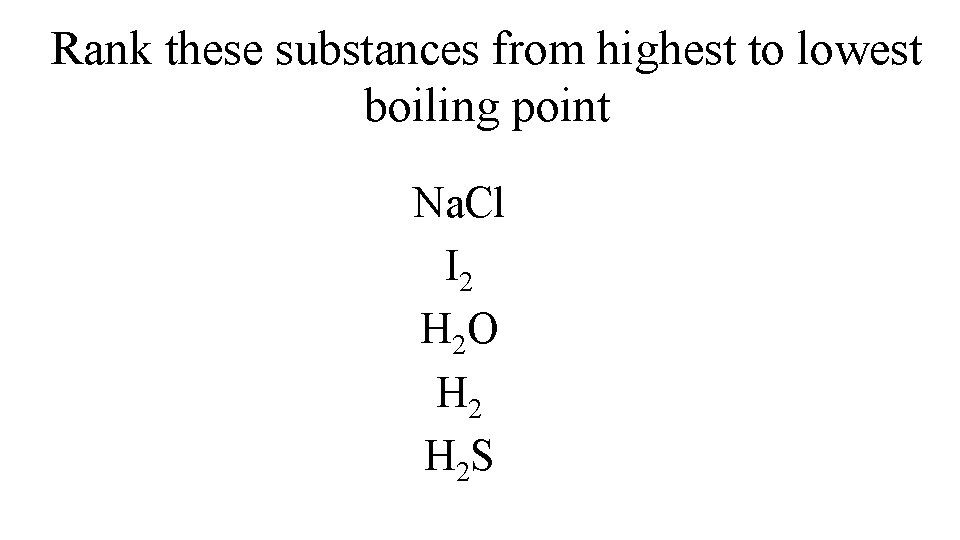
Rank these substances from highest to lowest boiling point Na. Cl I 2 H 2 O H 2 S

Highest Lowest Na. Cl Ionic bonding H 2 O H 2 S I 2 Hydrogen bonding IMF dipole-dipole IMF not as strong as H bonds stronger LDF larger e- cloud than H 2 weakest LDF smallest e- cloud

The End
- Slides: 11