Chemical Bonding I Chemical Bond attractive force between
Chemical Bonding I

Chemical Bond § attractive force between atoms or ions that binds them together as a unit § bonds form in order to… Ø decrease potential energy (PE) Ø increase stability
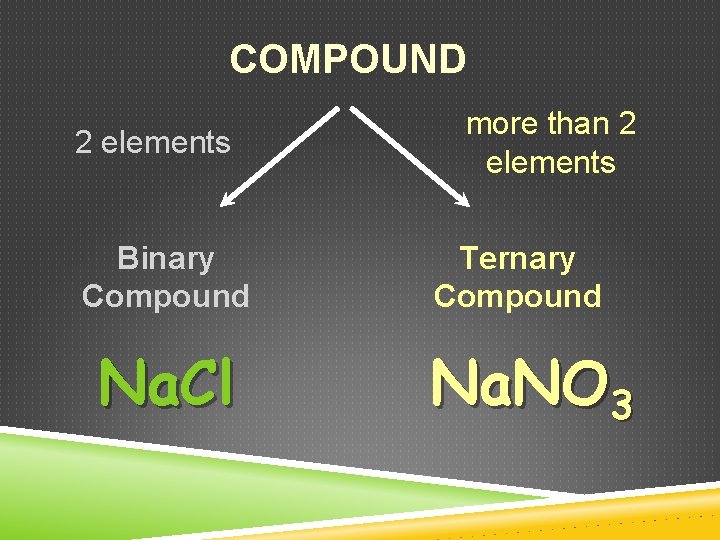
COMPOUND 2 elements Binary Compound Na. Cl more than 2 elements Ternary Compound Na. NO 3
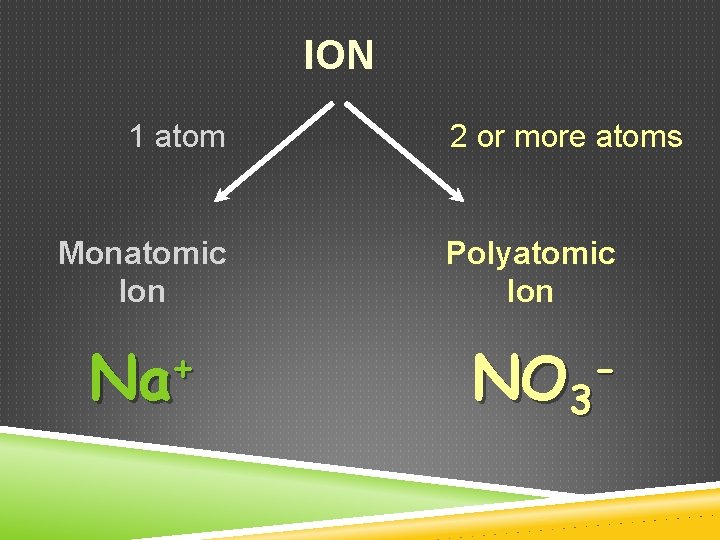
ION 1 atom Monatomic Ion + Na 2 or more atoms Polyatomic Ion NO 3
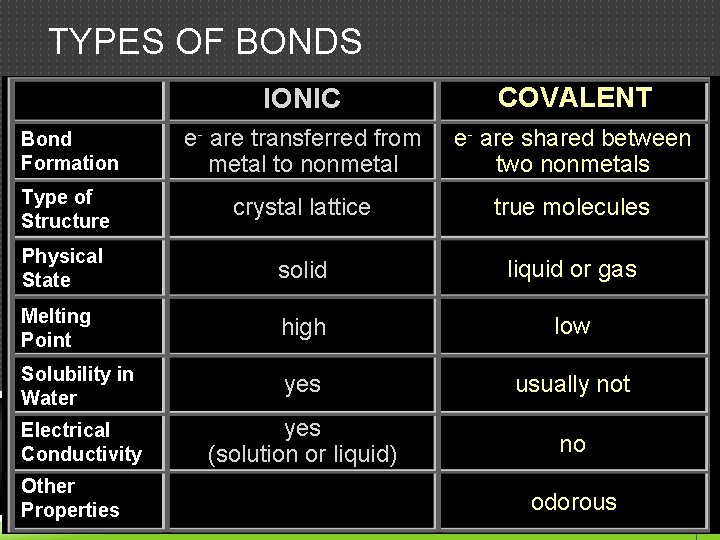
TYPES OF BONDS IONIC COVALENT Bond Formation e- are transferred from metal to nonmetal e- are shared between two nonmetals Type of Structure crystal lattice true molecules Physical State solid liquid or gas Melting Point high low Solubility in Water yes usually not Electrical Conductivity yes (solution or liquid) no Other Properties odorous
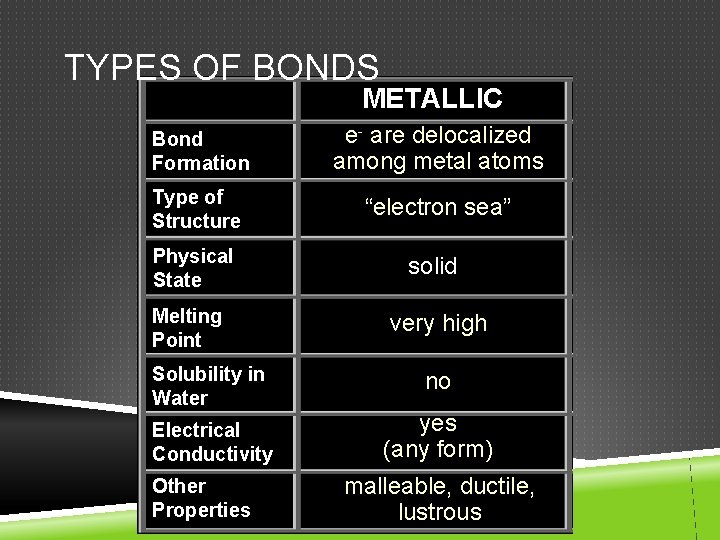
TYPES OF BONDS METALLIC Bond Formation e- are delocalized among metal atoms Type of Structure “electron sea” Physical State solid Melting Point very high Solubility in Water no Electrical Conductivity yes (any form) Other Properties malleable, ductile, lustrous
IONIC BONDS
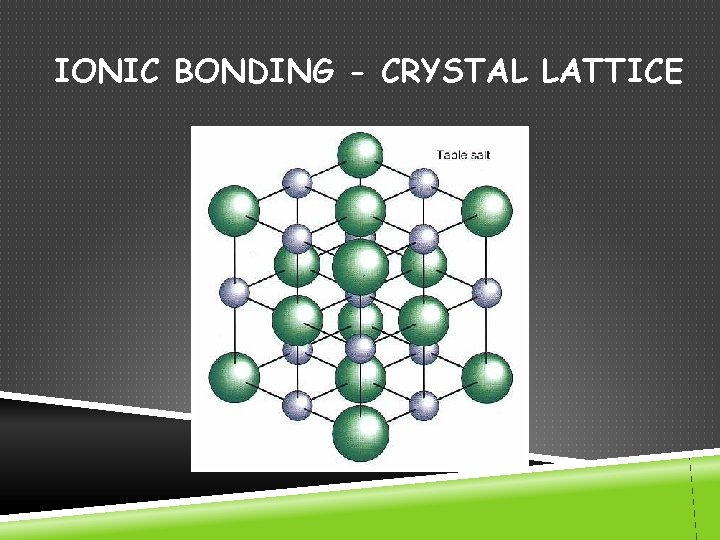
IONIC BONDING - CRYSTAL LATTICE
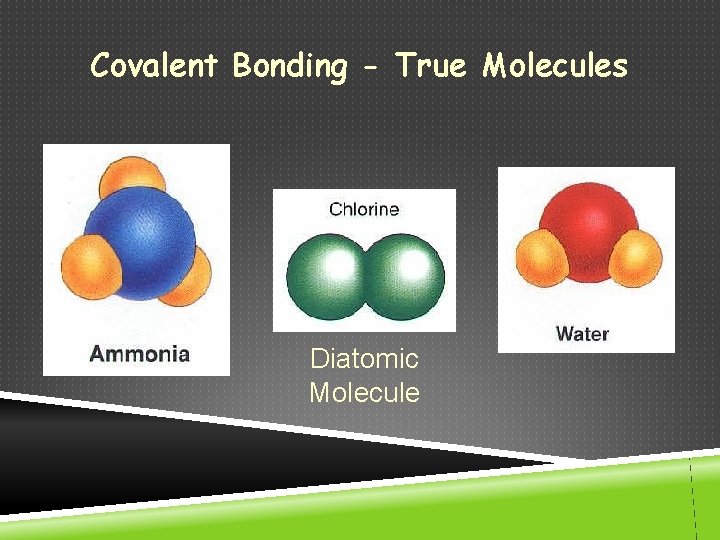
Covalent Bonding - True Molecules Diatomic Molecule
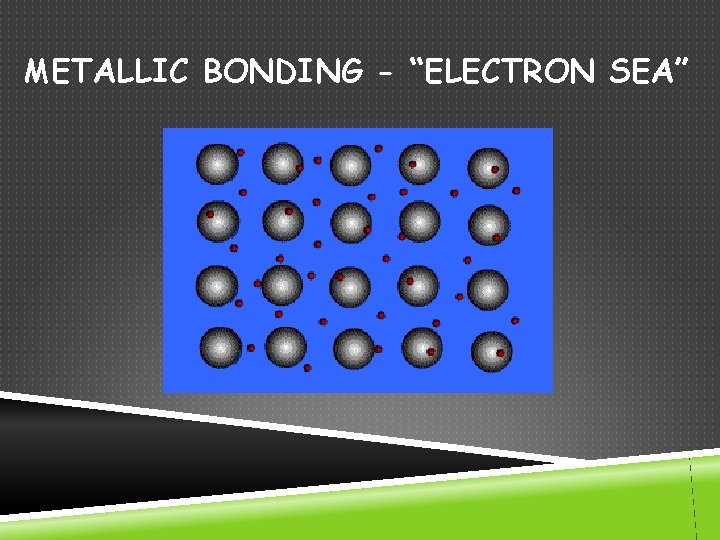
METALLIC BONDING - “ELECTRON SEA”
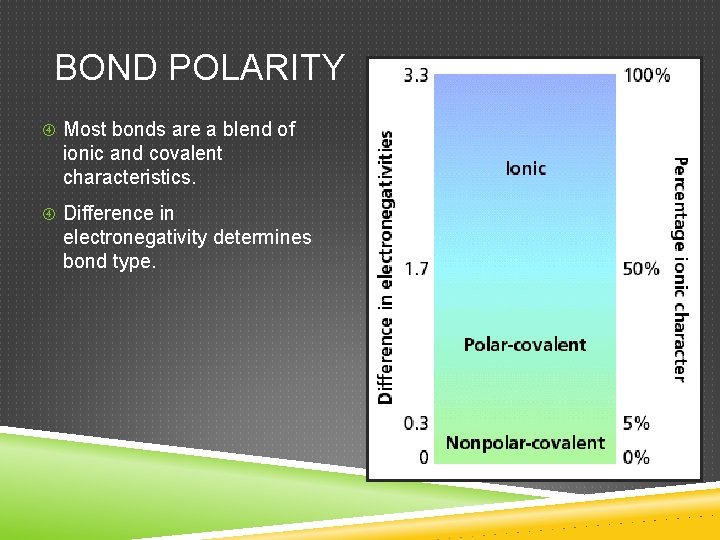
BOND POLARITY Most bonds are a blend of ionic and covalent characteristics. Difference in electronegativity determines bond type.
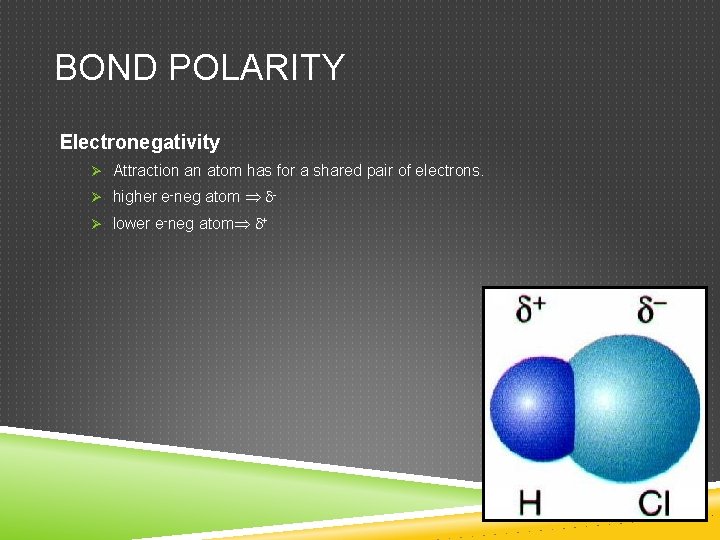
BOND POLARITY Electronegativity Ø Attraction an atom has for a shared pair of electrons. Ø higher e-neg atom Ø lower e-neg atom +
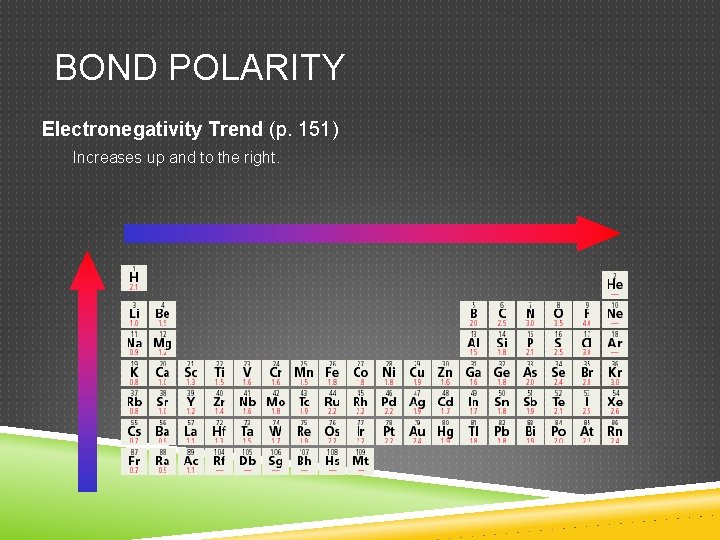
BOND POLARITY Electronegativity Trend (p. 151) Increases up and to the right.
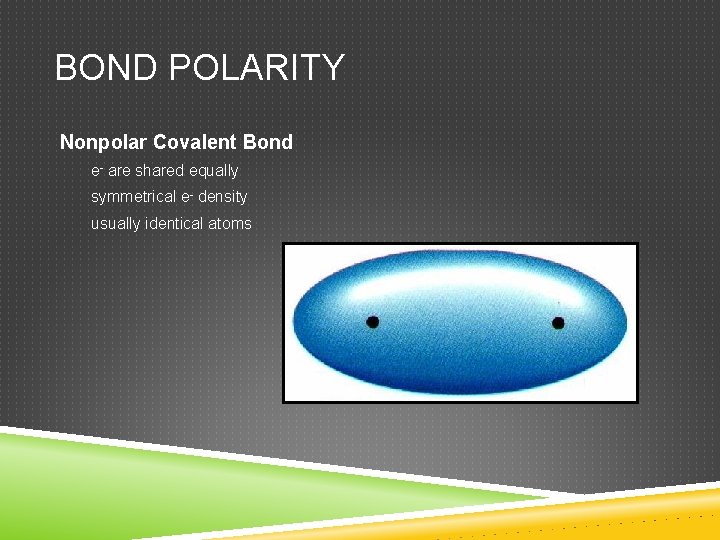
BOND POLARITY Nonpolar Covalent Bond e- are shared equally symmetrical e- density usually identical atoms
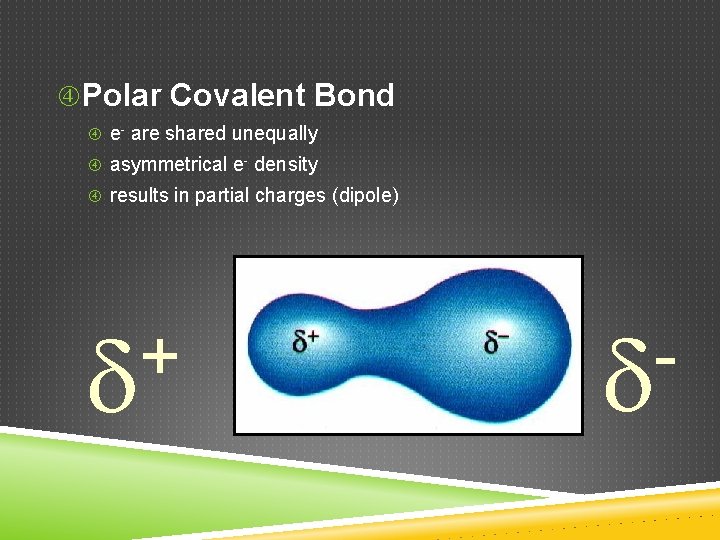
Polar Covalent Bond e- are shared unequally asymmetrical e- density results in partial charges (dipole) +
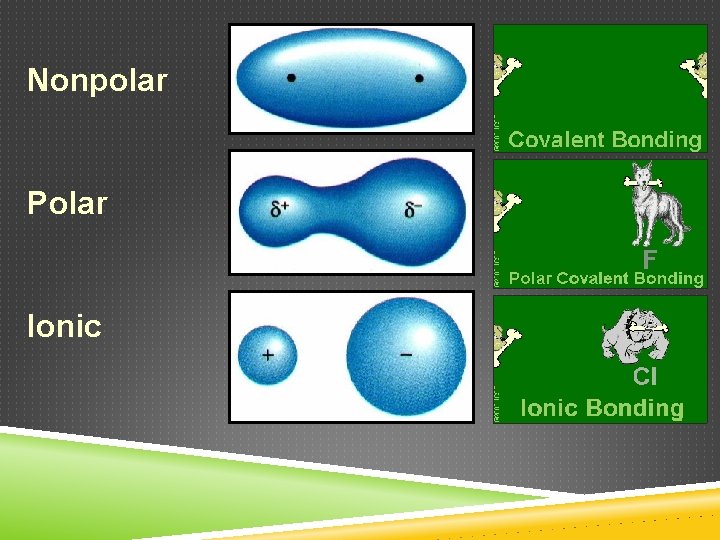
Nonpolar Polar Ionic
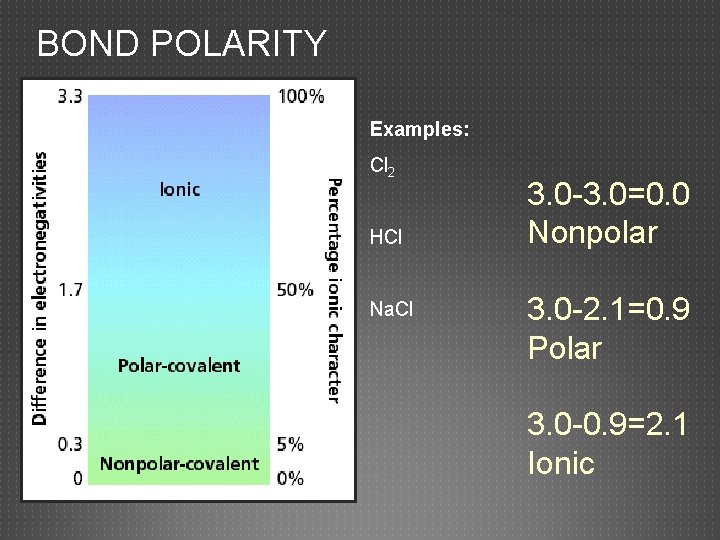
BOND POLARITY Examples: Cl 2 HCl Na. Cl 3. 0 -3. 0=0. 0 Nonpolar 3. 0 -2. 1=0. 9 Polar 3. 0 -0. 9=2. 1 Ionic

Chemical Bond § attractive force between atoms or ions that binds them together as a unit § bonds form in order to… Ø decrease potential energy (PE) Ø increase stability
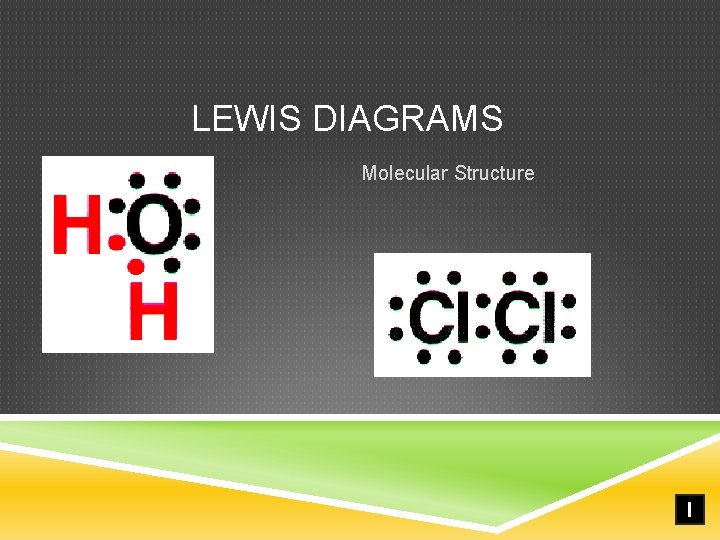
LEWIS DIAGRAMS Molecular Structure I

RULE Remember… Most atoms form bonds in order to have 8 valence electrons.
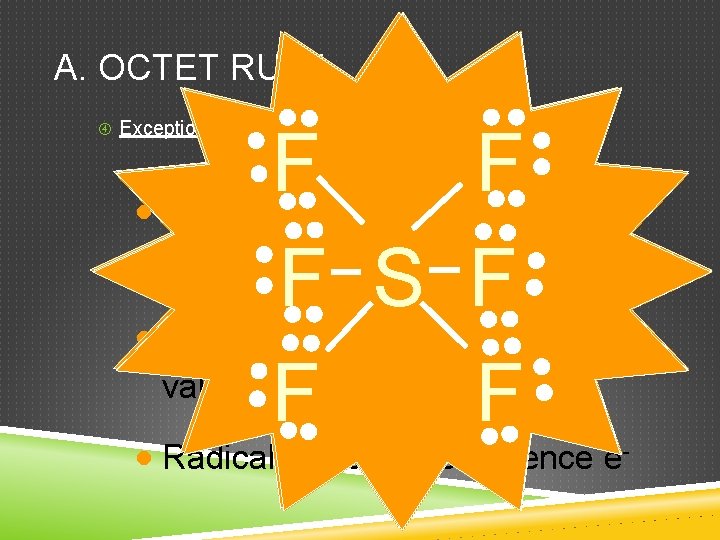
A. OCTET RULE Exceptions: F F · Hydrogen 2 valence e F B F · Groups F 1, 2, 3 get 2, 4, 6 valence e SO F H O H N · Expanded octet more than 8 valence e (e. g. S, P, Xe) Very unstable!! F F F - - · Radicals odd # of valence e- -
B. DRAWING LEWIS DIAGRAMS Find total # of valence e-. Arrange atoms - singular atom is usually in the middle. Form bonds between atoms (2 e-). Distribute remaining e- to give each atom an octet (recall exceptions). If there aren’t enough e- to go around, form double or triple bonds.
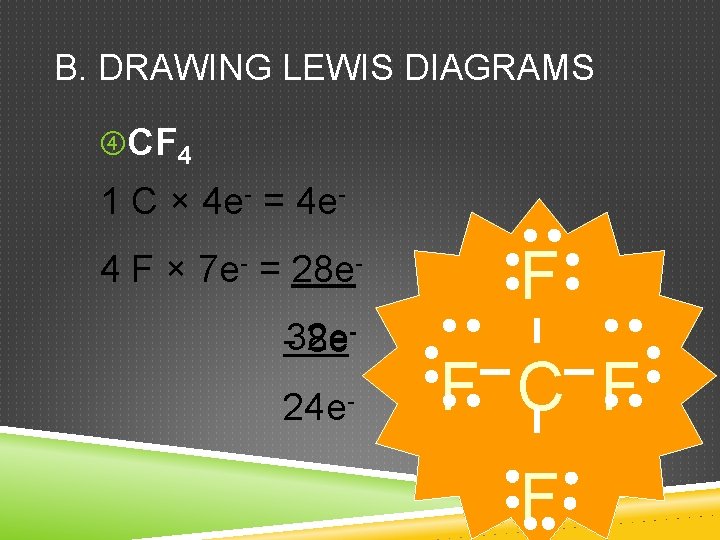
B. DRAWING LEWIS DIAGRAMS CF 4 1 C × 4 e- = 4 e 4 F × 7 e- = 28 e-32 e 8 e 24 e- F F C F F

B. DRAWING LEWIS DIAGRAMS Be. Cl 2 1 Be × 2 e- = 2 e 2 Cl × 7 e- = 14 e-16 e 4 e 12 e- Cl Be Cl
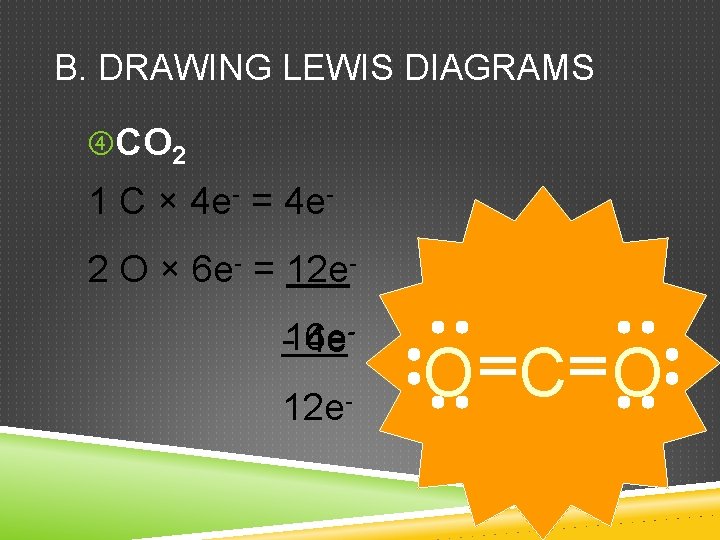
B. DRAWING LEWIS DIAGRAMS CO 2 1 C × 4 e- = 4 e 2 O × 6 e- = 12 e-16 e 4 e 12 e- O C O
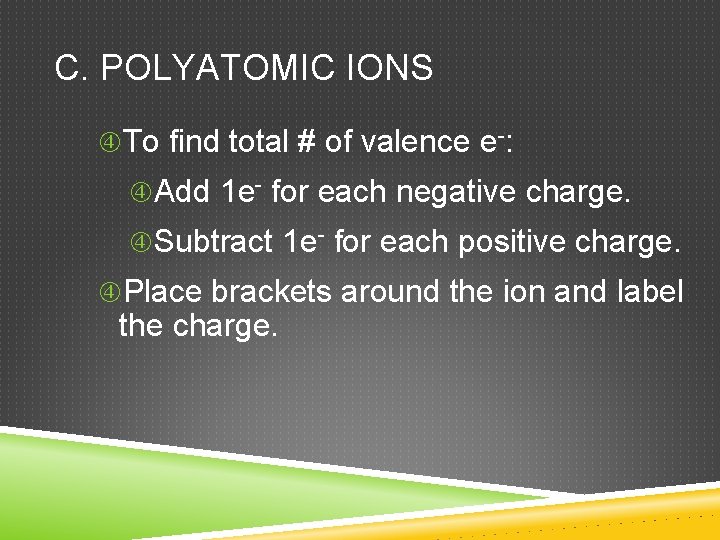
C. POLYATOMIC IONS To find total # of valence e-: Add 1 e- for each negative charge. Subtract 1 e- for each positive charge. Place brackets around the ion and label the charge.
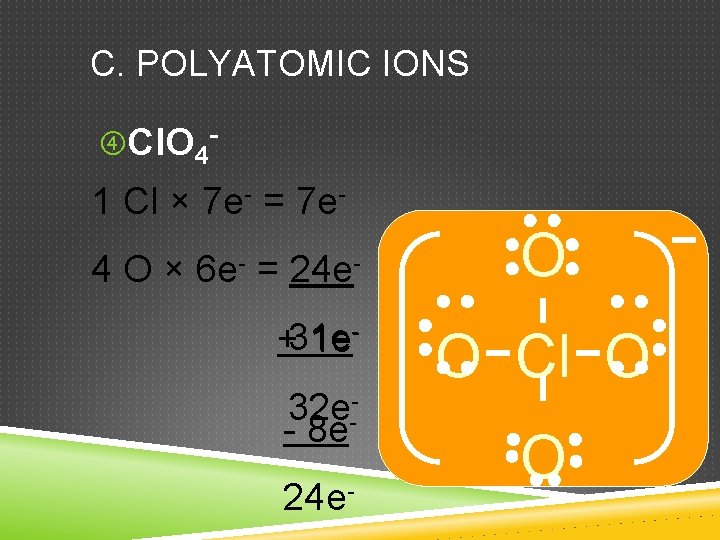
C. POLYATOMIC IONS Cl. O 4 - 1 Cl × 7 e- = 7 e 4 O × 6 e- = 24 e+31 e 1 e 32 e-- 8 e 24 e- O O Cl O O
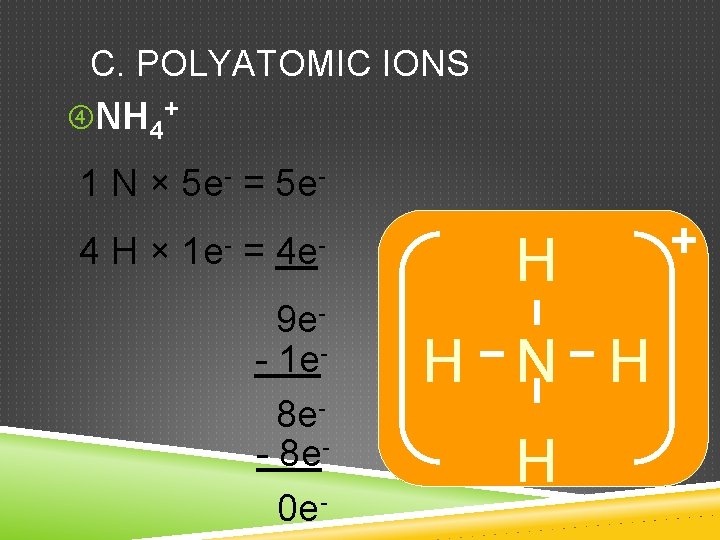
C. POLYATOMIC IONS NH 4+ 1 N × 5 e- = 5 e 4 H × 1 e- = 4 e 9 e- 1 e 8 e- 8 e 0 e- H H N H H
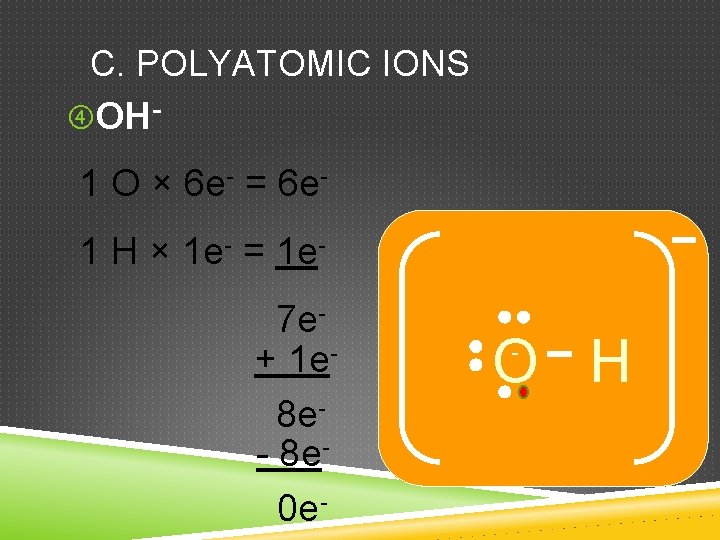
C. POLYATOMIC IONS OH- 1 O × 6 e- = 6 e 1 H × 1 e- = 1 e 7 e+ 1 e 8 e- 8 e 0 e- O H
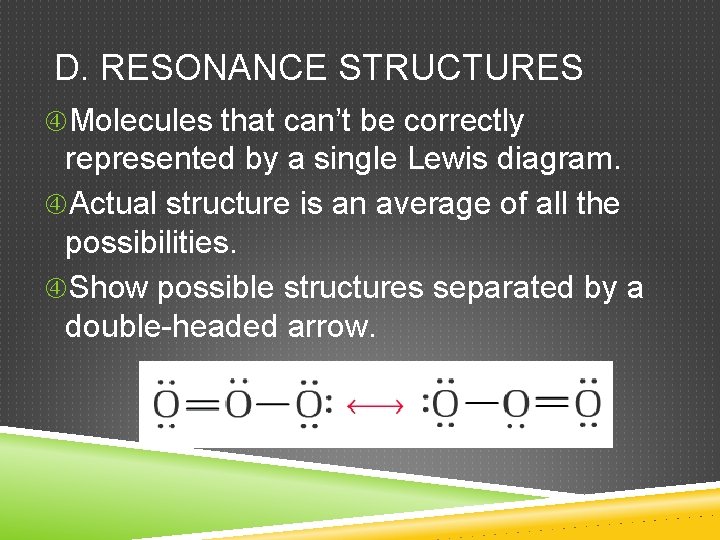
D. RESONANCE STRUCTURES Molecules that can’t be correctly represented by a single Lewis diagram. Actual structure is an average of all the possibilities. Show possible structures separated by a double-headed arrow.
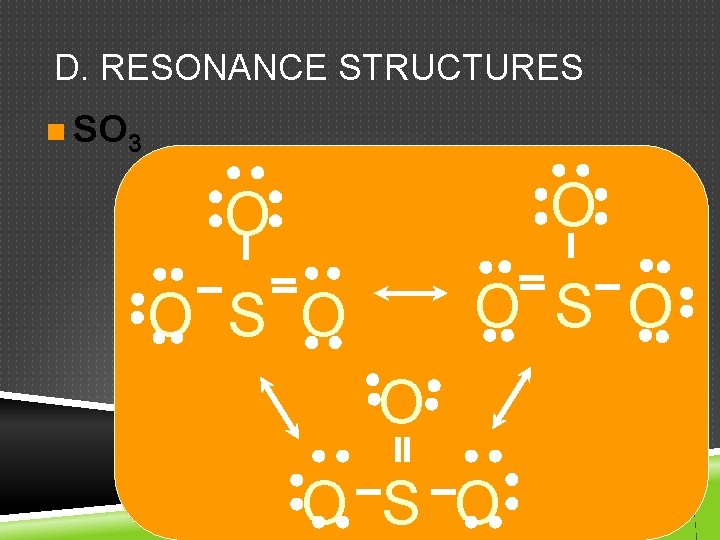
D. RESONANCE STRUCTURES n SO 3 O O O S O
MOLECULAR GEOMETRY I
VSEPR THEORY Valence Shell Electron Pair Repulsion Theory Electron pairs orient themselves in order to minimize repulsive forces.

VSEPR THEORY Types of e- Pairs Bonding pairs - form bonds Lone pairs - nonbonding e- Lone pairs repel more strongly than bonding pairs!!!
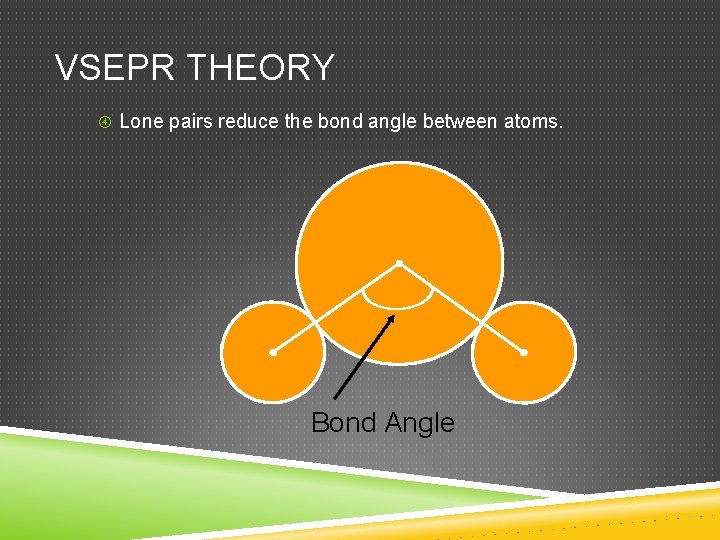
VSEPR THEORY Lone pairs reduce the bond angle between atoms. Bond Angle
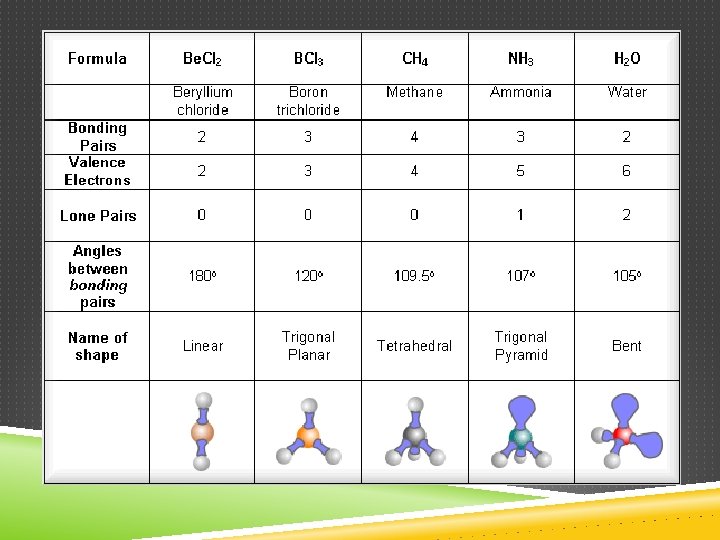
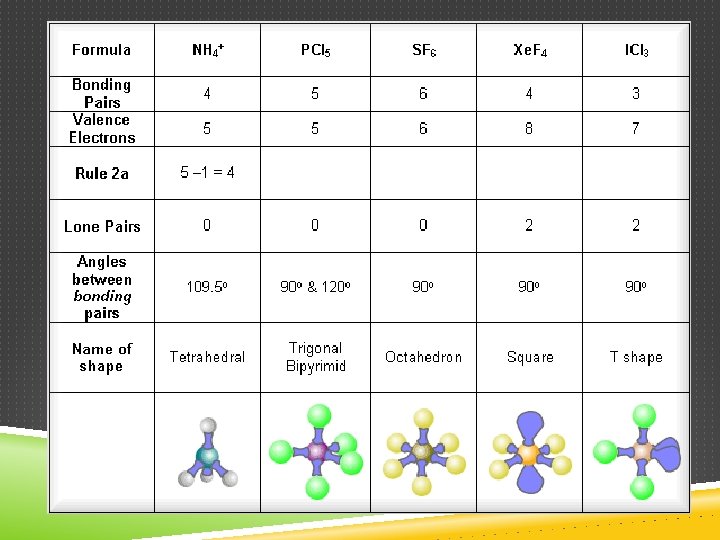
DETERMINING MOLECULAR SHAPE Draw the Lewis Diagram. Tally up e- pairs on central atom. double/triple bonds = ONE pair Shape is determined by the # of bonding pairs and lone pairs. Know the 8 common shapes & their bond angles!
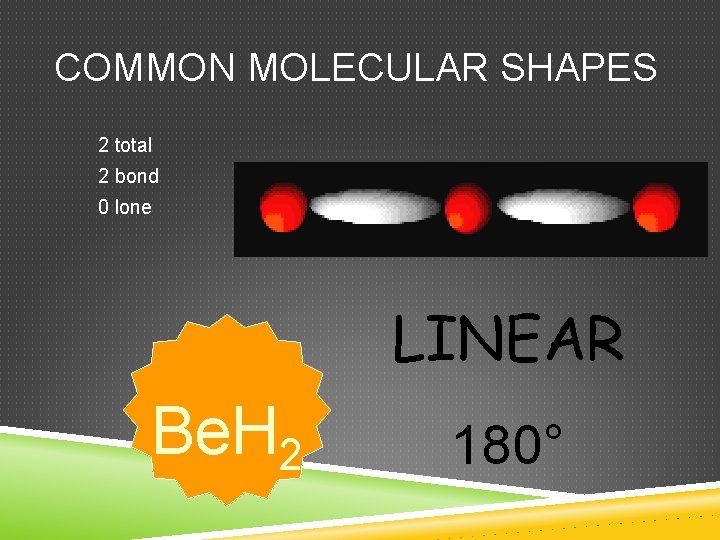
COMMON MOLECULAR SHAPES 2 total 2 bond 0 lone LINEAR Be. H 2 180°
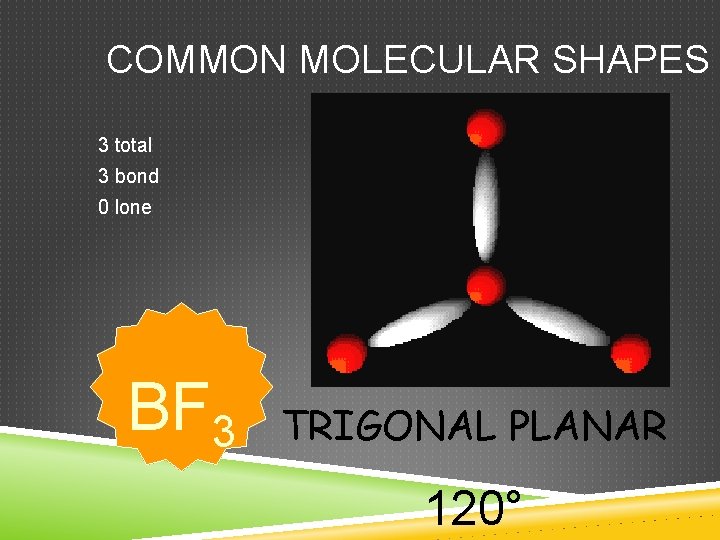
COMMON MOLECULAR SHAPES 3 total 3 bond 0 lone BF 3 TRIGONAL PLANAR 120°
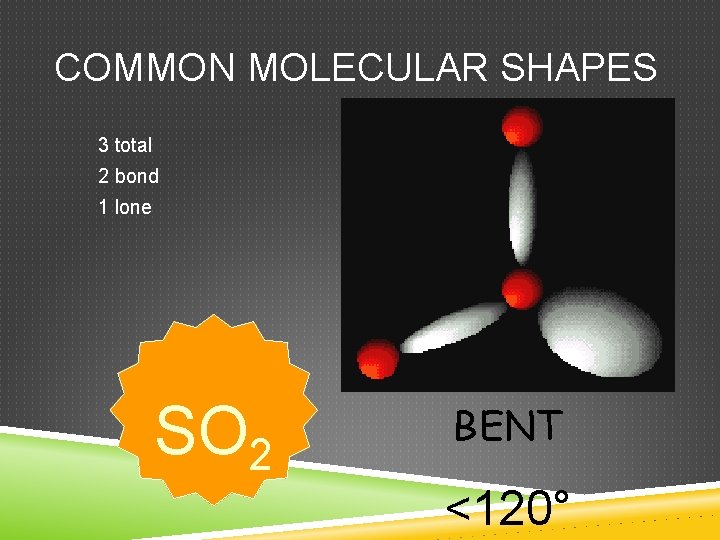
COMMON MOLECULAR SHAPES 3 total 2 bond 1 lone SO 2 BENT <120°
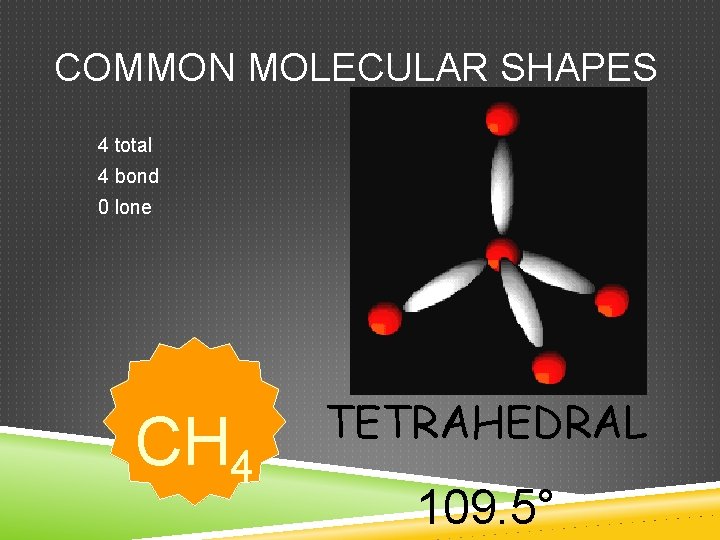
COMMON MOLECULAR SHAPES 4 total 4 bond 0 lone CH 4 TETRAHEDRAL 109. 5°
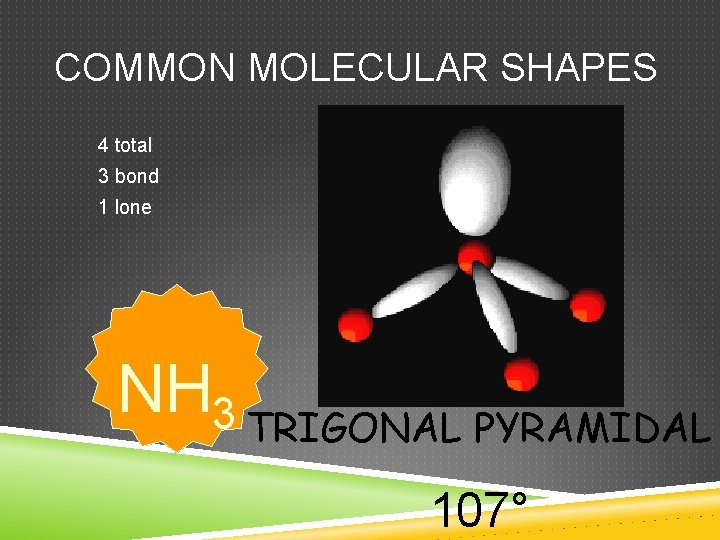
COMMON MOLECULAR SHAPES 4 total 3 bond 1 lone NH 3 TRIGONAL PYRAMIDAL 107°
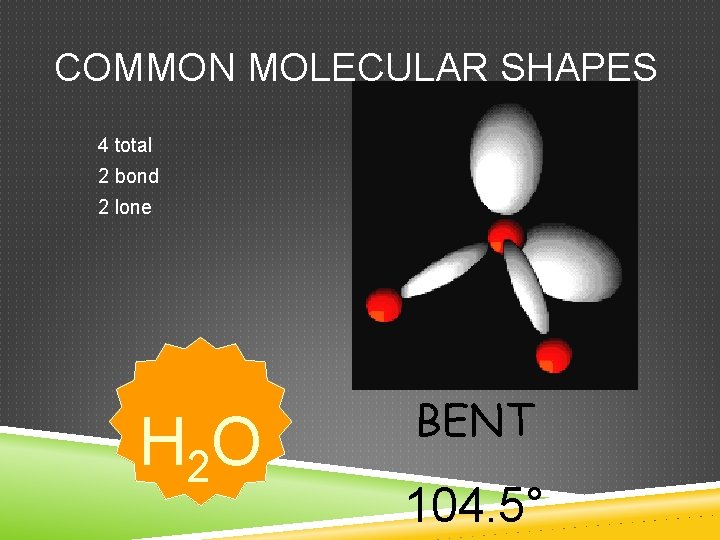
COMMON MOLECULAR SHAPES 4 total 2 bond 2 lone H 2 O BENT 104. 5°

COMMON MOLECULAR SHAPES 5 total 5 bond 0 lone PCl 5 TRIGONAL BIPYRAMIDAL 120°/90°
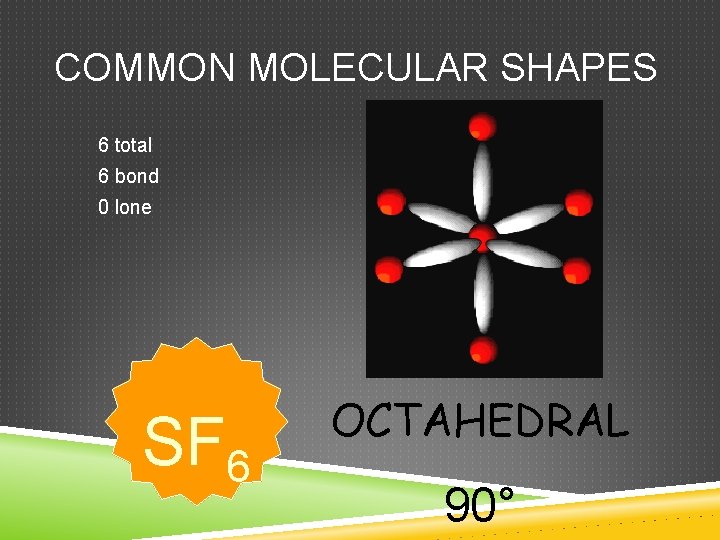
COMMON MOLECULAR SHAPES 6 total 6 bond 0 lone SF 6 OCTAHEDRAL 90°
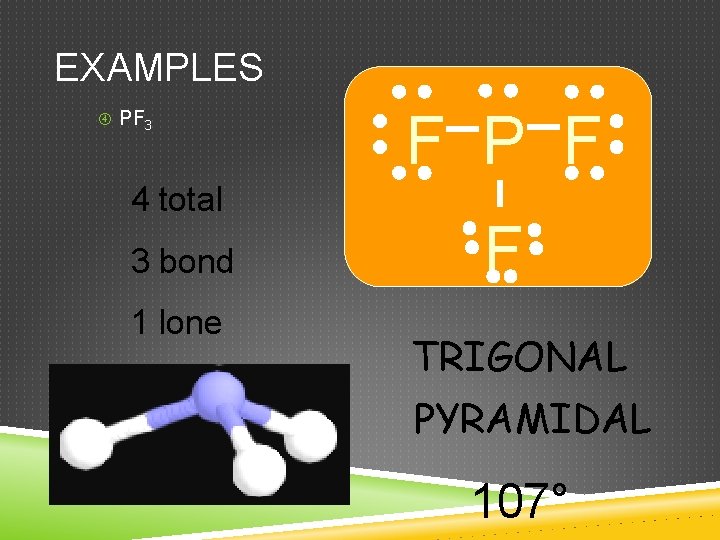
EXAMPLES PF 3 4 total 3 bond 1 lone F P F F TRIGONAL PYRAMIDAL 107°
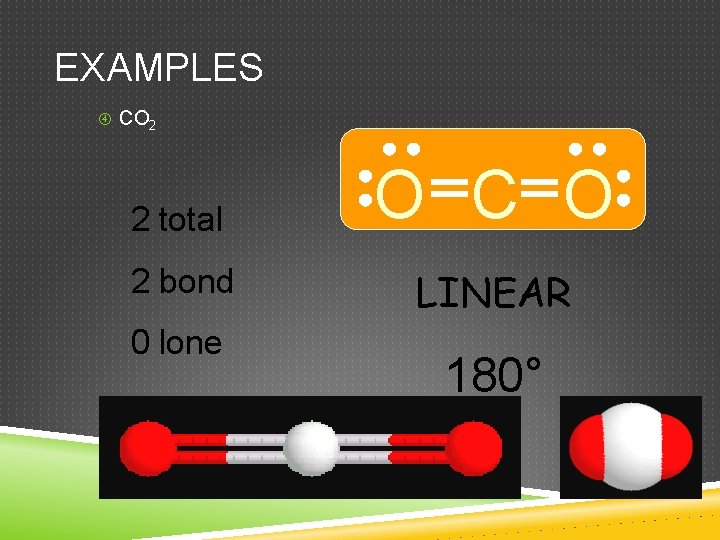
EXAMPLES CO 2 2 total 2 bond 0 lone O C O LINEAR 180°
- Slides: 48