Chemical Bonding Covalent Bonds Formed when 2 atoms

Chemical Bonding

Covalent Bonds Formed when 2 atoms share electrons

Polar Covalent Bonds n n Unequal sharing of electrons Electrons attracted more to atom with the higher electronegativity Electronegativity difference is between 0. 4 – 1. 7 Polar Covalent Bonds dissolve in water
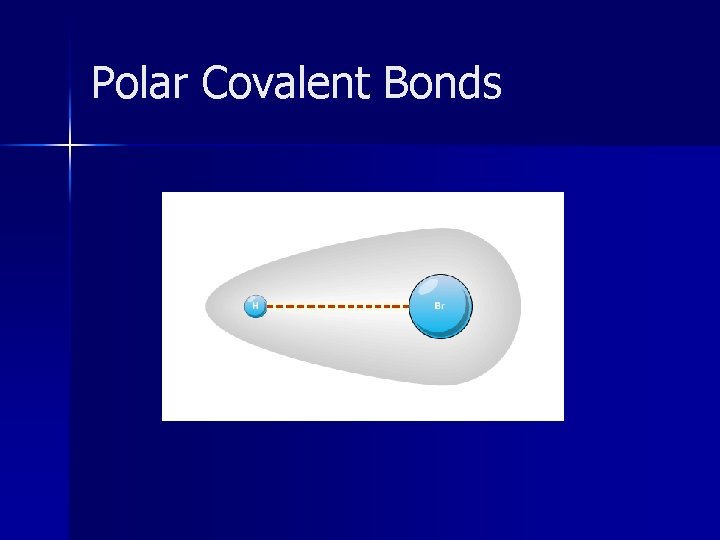
Polar Covalent Bonds

Non-polar Covalent Bonds n n Equal sharing of electrons Electronegativity difference is between basically 0. Usually found between diatomic molecules – two of the same atoms Non-polar covalent bonds don’t dissolve in water.

Non-polar Covalent Bonds

Ionic Bonds Electrical attraction between large numbers of cations and anions n Ion = a charged particle n Cation = + charged particle n – Loves to give electrons away – Low Ionization Energy & Electronegativity – Typically are metals
Ionic Bonds (cont. ) n Anion = - charged particle – Loves to accept electrons – High Ionization Energy & Electronegativity – Typically are nonmetals n Electronegativity difference >1. 7
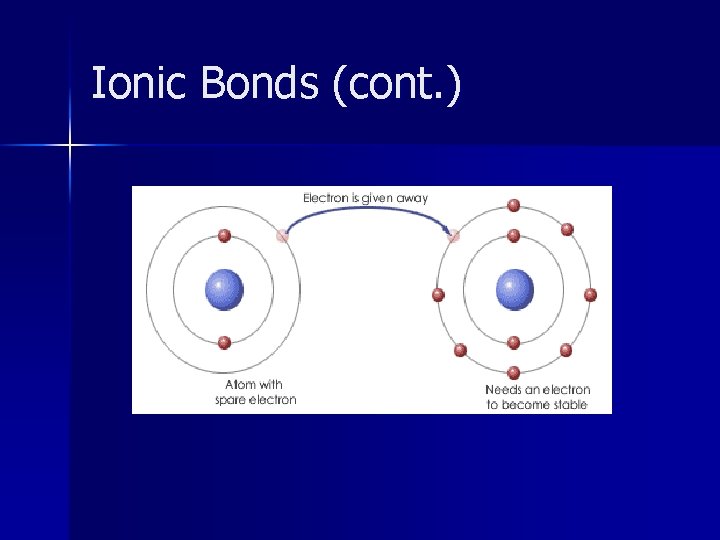
Ionic Bonds (cont. )

Ionic Bonds (cont. ) n If a bond is ionic: – The compound will fall apart (dissociate) into its original ions when dissolved in water – If a conductivity test is performed, the light bulb will… light!

Metallic Bonds Bond formed between multiple metal atoms. n Electrons are free to move within electron clouds of all metal ions = Sea of Electrons n Electrons are delocalized – they don’t belong to any one atom anymore. n
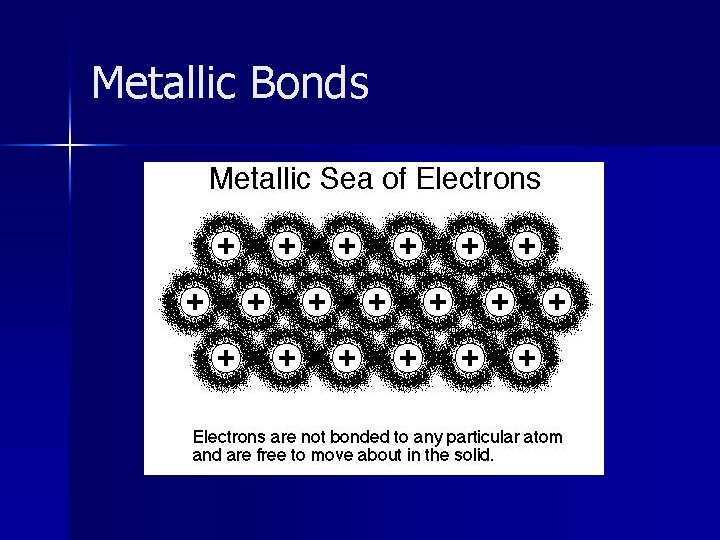
Metallic Bonds
Hydrogen Bonds Intermolecular – attraction between molecules n One molecule is polar and has Hydrogen in a H-F, H-O or H-N bond n Other molecule has an unshared pair of electrons, usually F, O, N n
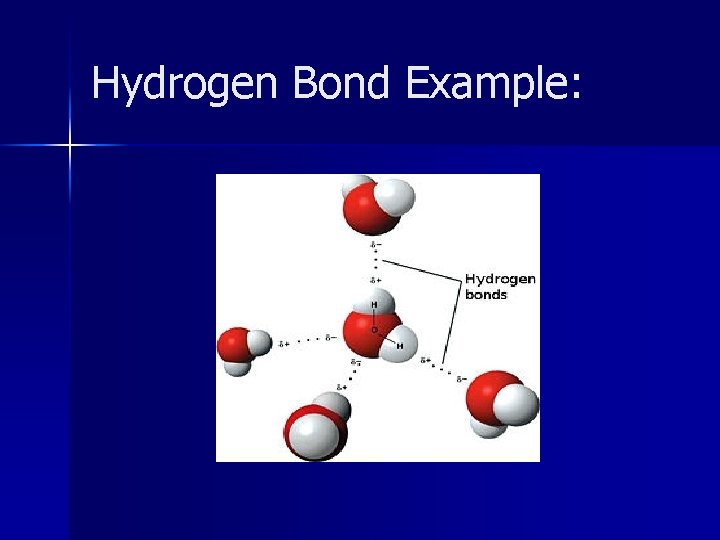
Hydrogen Bond Example:

Van der Waals Bonds These bonds are important for Noble Gases n Very weak bonds n Electrons of Noble Gases get shifted to one side causing it to attract a slightly positive atom that is nearby. n
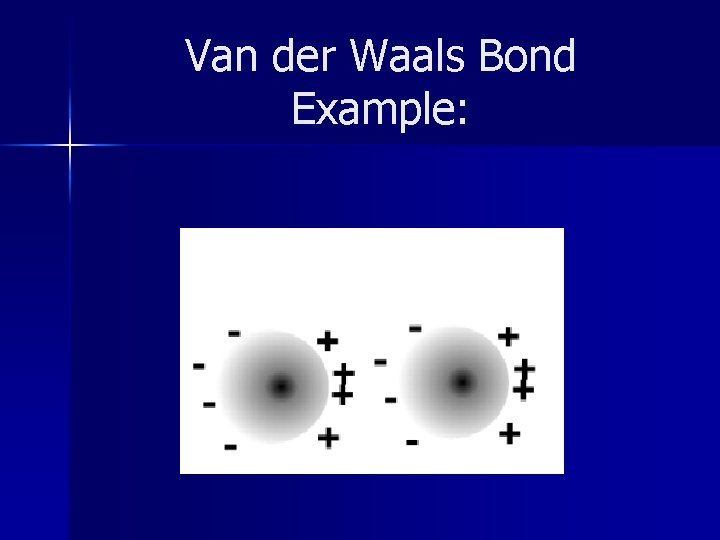
Van der Waals Bond Example:
- Slides: 16