Chemical Bonding Classifying Chemical Compounds Bond Types Atoms

Chemical Bonding Classifying Chemical Compounds
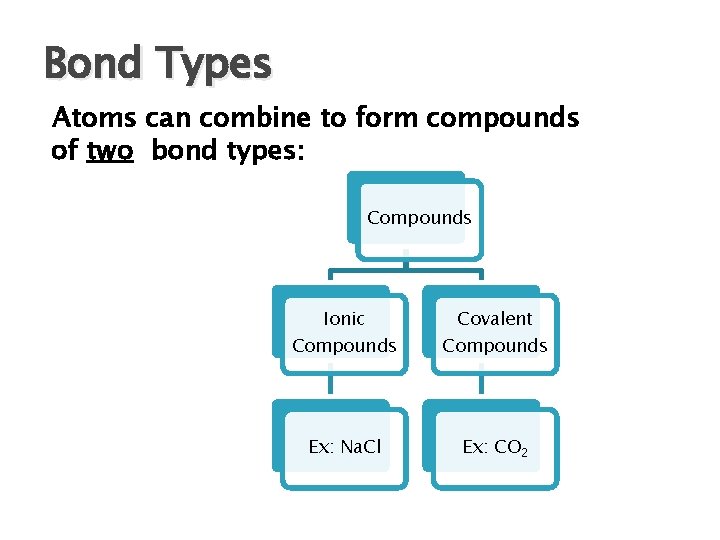
Bond Types Atoms can combine to form compounds of two bond types: Compounds Ionic Covalent Compounds Ex: Na. Cl Ex: CO 2
Physical Properties – Comparison Property State at room temperature Melting point Electrical conductivity as a liquid Solubility in water Electrical conductivity when dissolved in water Ionic Compound Covalent Compound
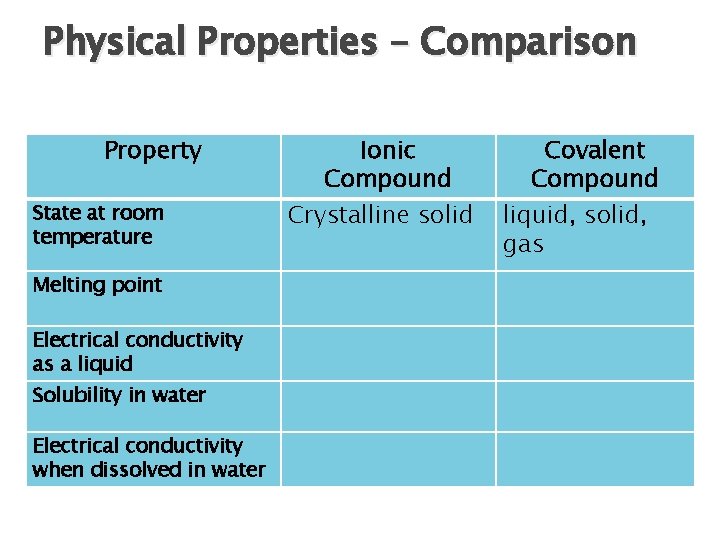
Physical Properties – Comparison Property State at room temperature Melting point Electrical conductivity as a liquid Solubility in water Electrical conductivity when dissolved in water Ionic Compound Crystalline solid Covalent Compound liquid, solid, gas
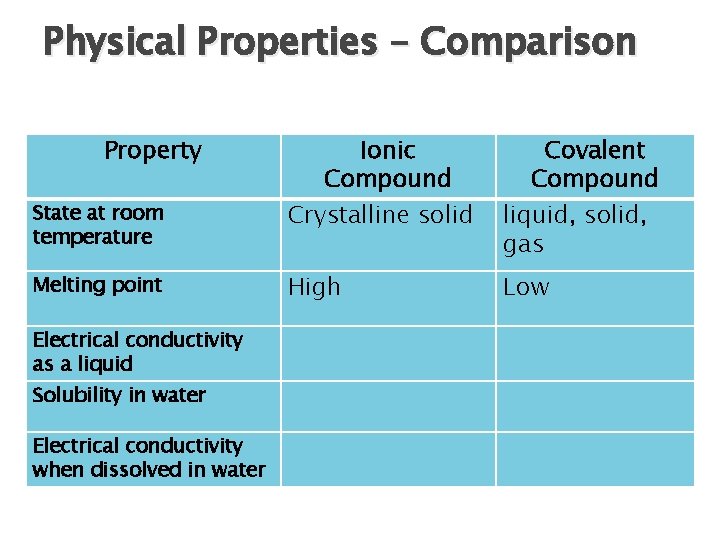
Physical Properties – Comparison Property State at room temperature Melting point Electrical conductivity as a liquid Solubility in water Electrical conductivity when dissolved in water Ionic Compound Crystalline solid Covalent Compound liquid, solid, gas High Low
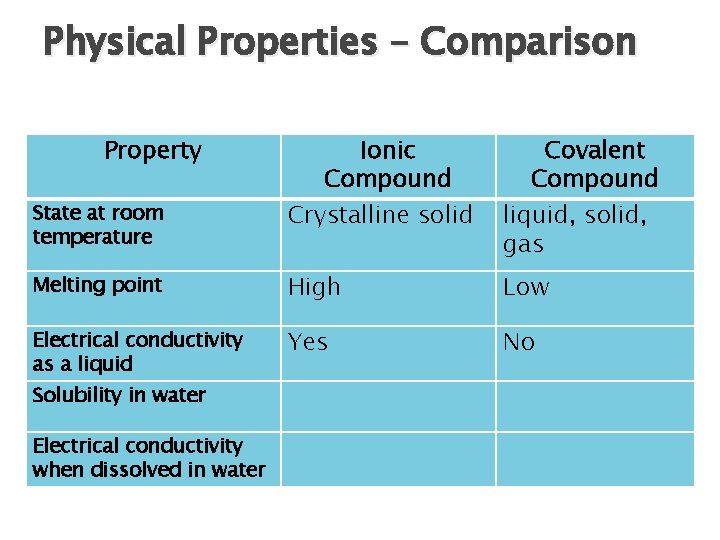
Physical Properties – Comparison Property Ionic Compound Crystalline solid Covalent Compound liquid, solid, gas Melting point High Low Electrical conductivity as a liquid Yes No State at room temperature Solubility in water Electrical conductivity when dissolved in water
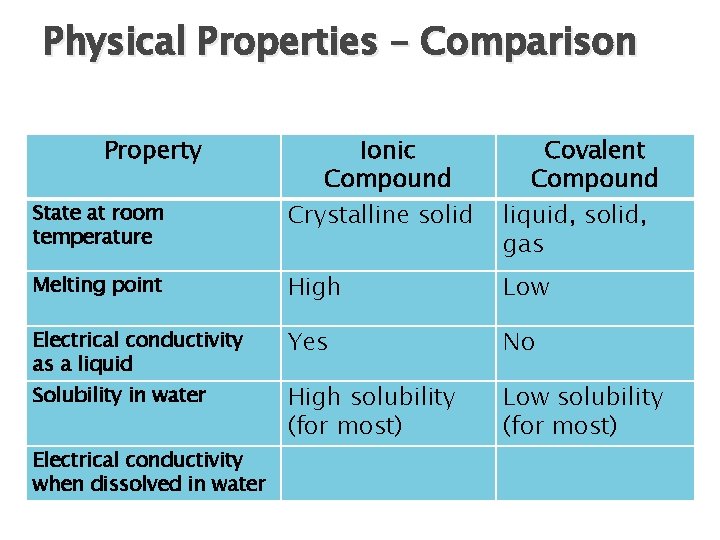
Physical Properties – Comparison Property Ionic Compound Crystalline solid Covalent Compound liquid, solid, gas Melting point High Low Electrical conductivity as a liquid Yes No High solubility (for most) Low solubility (for most) State at room temperature Solubility in water Electrical conductivity when dissolved in water
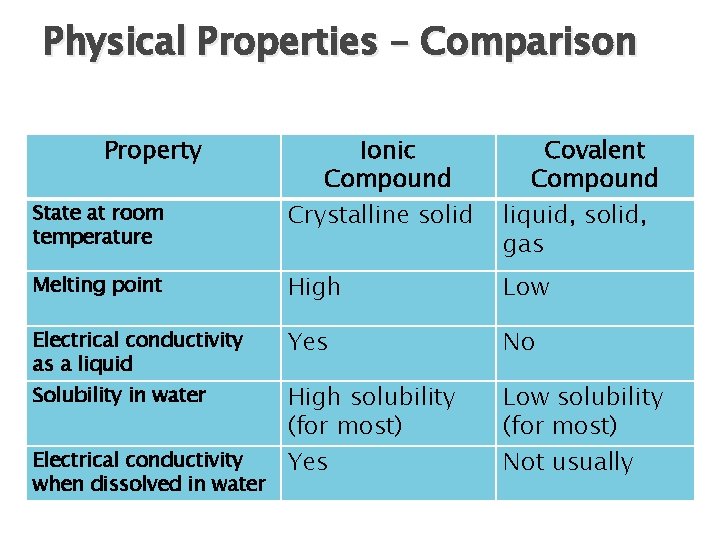
Physical Properties – Comparison Property Ionic Compound Crystalline solid Covalent Compound liquid, solid, gas Melting point High Low Electrical conductivity as a liquid Yes No High solubility (for most) Yes Low solubility (for most) Not usually State at room temperature Solubility in water Electrical conductivity when dissolved in water
Chemical Properties – Comparison Bonds form when the valence e- of atoms interact. Atoms can either exchange eor share e. There are two types of chemical bonds
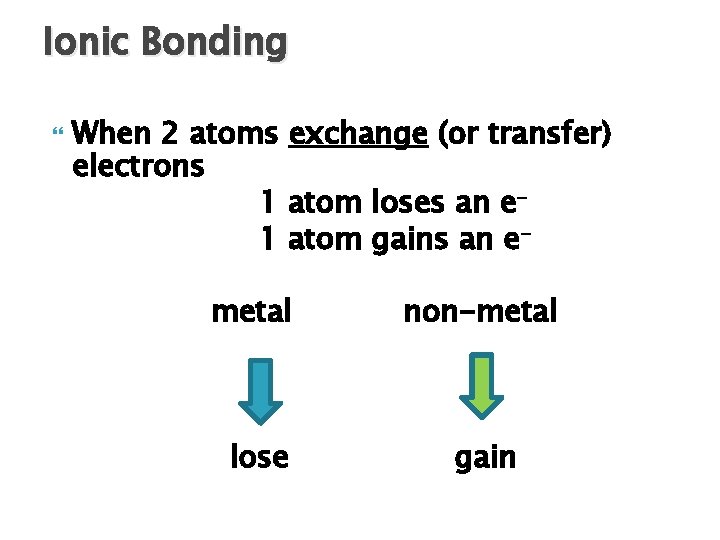
Ionic Bonding When 2 atoms exchange (or transfer) electrons 1 atom loses an e 1 atom gains an emetal non-metal lose gain
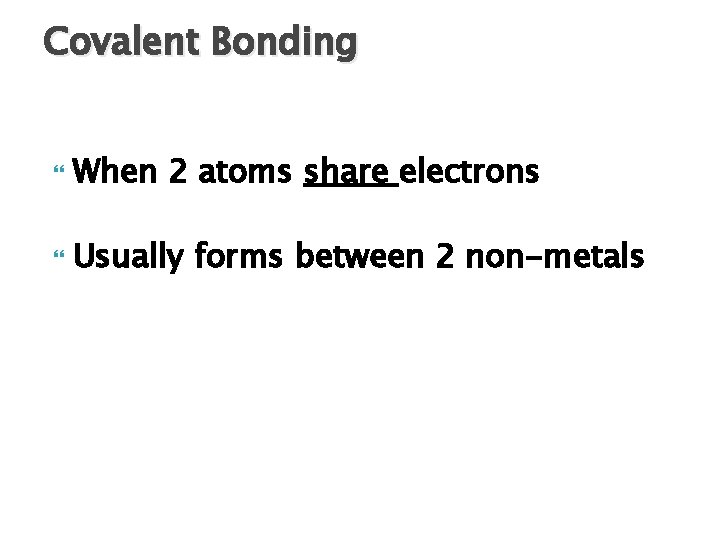
Covalent Bonding When 2 atoms share electrons Usually forms between 2 non-metals

Electronegativity Recall: Electronegativity (EN) is a measure of an atom’s ability to attract electrons in a chemical bond. When 2 atoms form a bond, each atom attracts the other’s electrons in addition to its own.
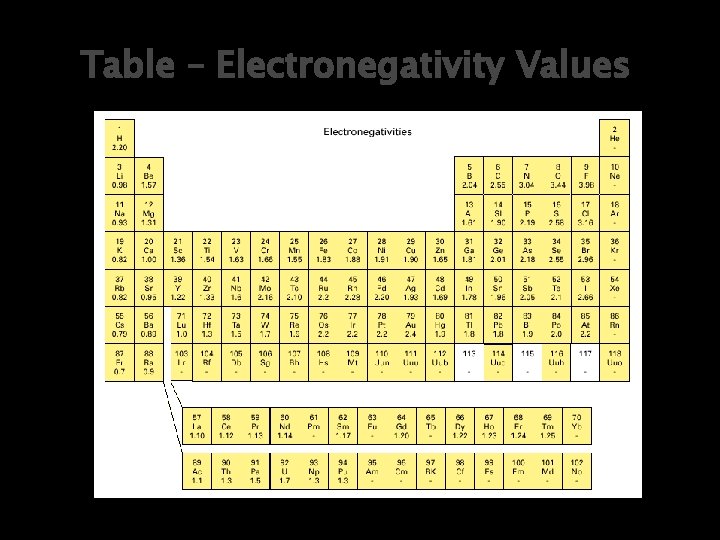
Table – Electronegativity Values
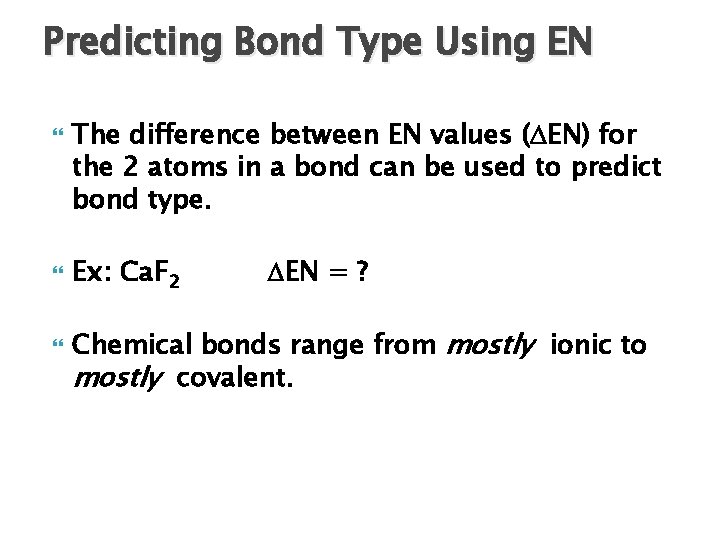
Predicting Bond Type Using EN The difference between EN values ( EN) for the 2 atoms in a bond can be used to predict bond type. Ex: Ca. F 2 EN = ? Chemical bonds range from mostly ionic to mostly covalent.
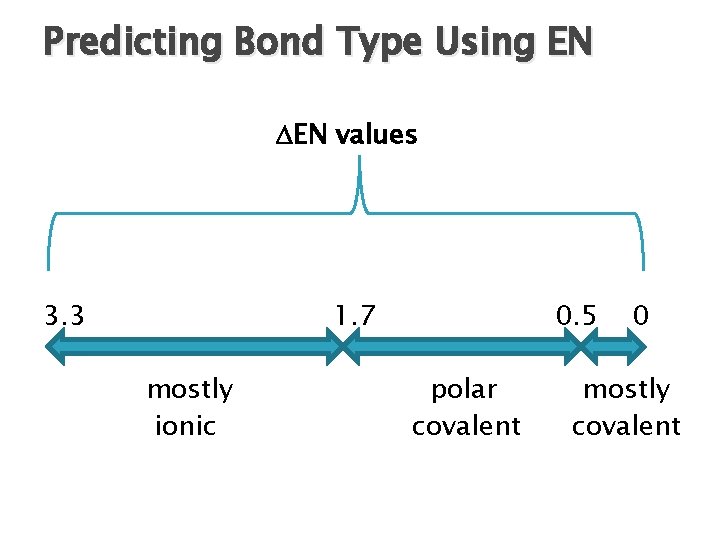
Predicting Bond Type Using EN EN values 3. 3 1. 7 mostly ionic 0. 5 polar covalent 0 mostly covalent
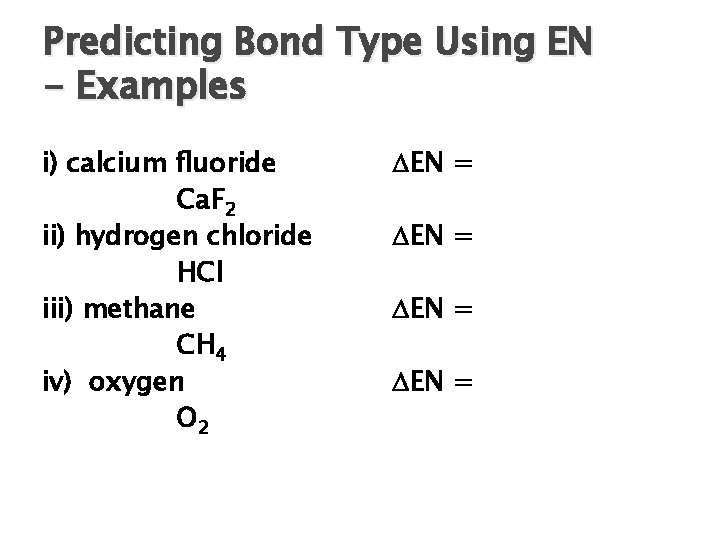
Predicting Bond Type Using EN - Examples i) calcium fluoride Ca. F 2 ii) hydrogen chloride HCl iii) methane CH 4 iv) oxygen O 2 EN =

Chemical Bonding Bond Formation

Covalent Bonding Involves sharing of e Sharing of e- between atoms may not be equal. non-polar covalent ◦ (mostly covalent) bond involves equal sharing polar covalent ◦ bond involves unequal sharing
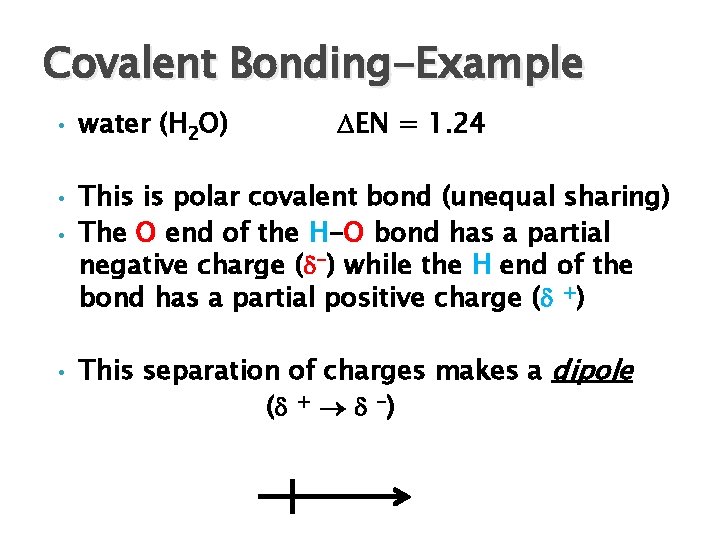
Covalent Bonding-Example • • water (H 2 O) EN = 1. 24 This is polar covalent bond (unequal sharing) The O end of the H-O bond has a partial negative charge ( -) while the H end of the bond has a partial positive charge ( +) This separation of charges makes a dipole ( + -)
Polar Bonds • • Using EN values you can predict the polarity of a bond. The overall polarity of a molecule depends on a combination of: § the polarity of the bonds § the shape of the molecule
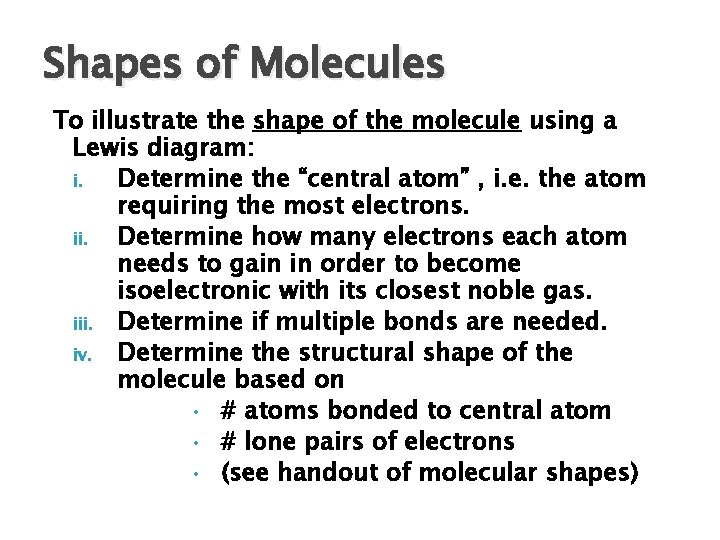
Shapes of Molecules To illustrate the shape of the molecule using a Lewis diagram: i. Determine the “central atom” , i. e. the atom requiring the most electrons. ii. Determine how many electrons each atom needs to gain in order to become isoelectronic with its closest noble gas. iii. Determine if multiple bonds are needed. iv. Determine the structural shape of the molecule based on • # atoms bonded to central atom • # lone pairs of electrons • (see handout of molecular shapes)

How Does That Look Again? Shapes of Molecules
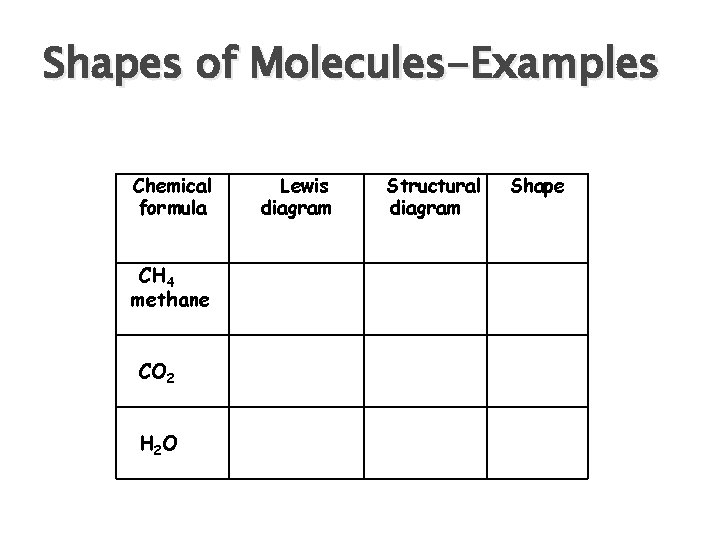
Shapes of Molecules-Examples Chemical formula CH 4 methane CO 2 H 2 O Lewis diagram Structural diagram Shape
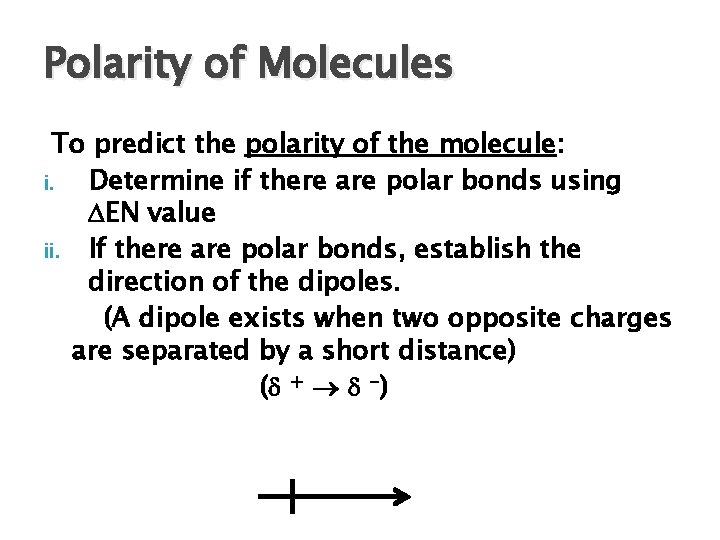
Polarity of Molecules To predict the polarity of the molecule: i. Determine if there are polar bonds using EN value ii. If there are polar bonds, establish the direction of the dipoles. (A dipole exists when two opposite charges are separated by a short distance) ( + -)
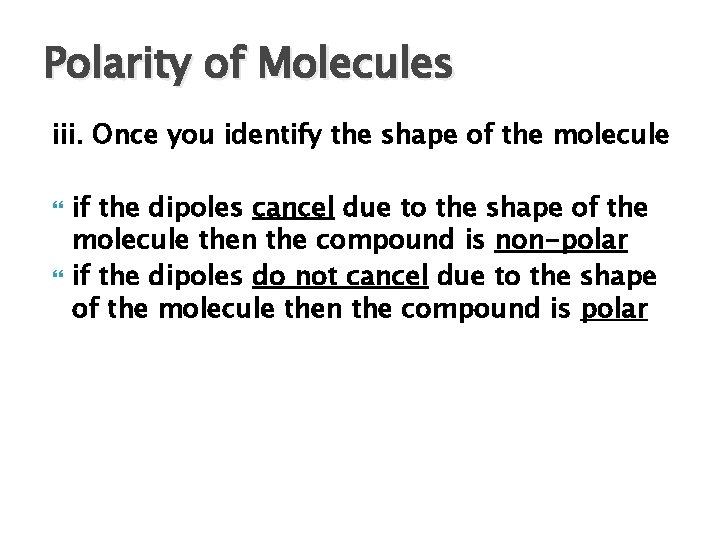
Polarity of Molecules iii. Once you identify the shape of the molecule if the dipoles cancel due to the shape of the molecule then the compound is non-polar if the dipoles do not cancel due to the shape of the molecule then the compound is polar
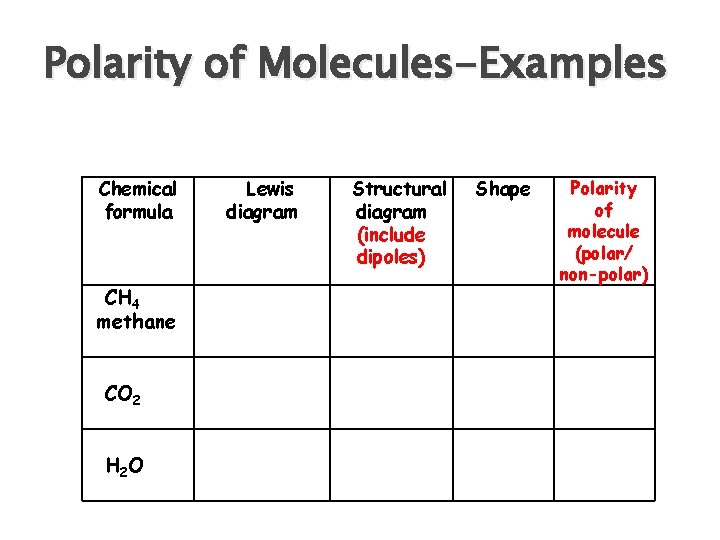
Polarity of Molecules-Examples Chemical formula CH 4 methane CO 2 H 2 O Lewis diagram Structural diagram (include dipoles) Shape Polarity of molecule (polar/ non-polar)

Video Clip Polar Bonding

Chemical Bonding Chemical Formulas - Ionic

Writing Formulas–Binary Compounds qcontain H, metal or metalloid with only 1 oxidation number (or valence) (see periodic table for values) qname the first element and change the ending (suffix) of second element to “ide” q. Use zero sum rule to write formula q. Try Table 1
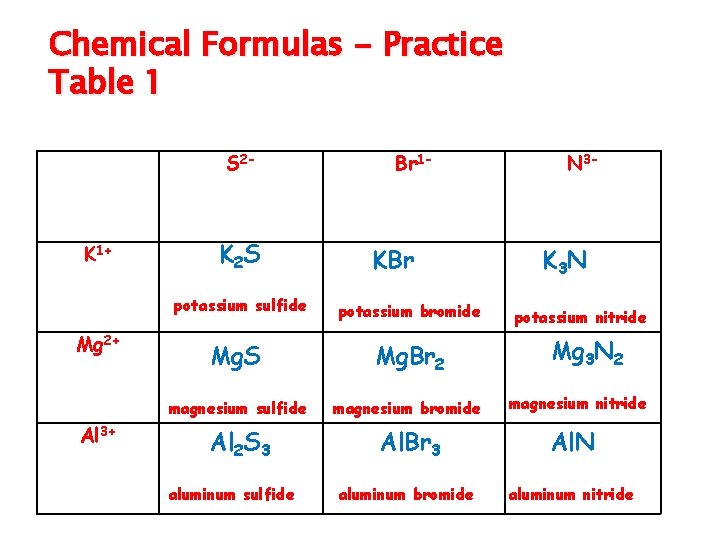
Chemical Formulas - Practice Table 1 S 2 - K 1+ K 2 S potassium sulfide Mg 2+ Al 3+ Br 1 - KBr potassium bromide N 3 - K 3 N potassium nitride Mg 3 N 2 Mg. S Mg. Br 2 magnesium sulfide magnesium bromide Al 2 S 3 Al. Br 3 Al. N aluminum bromide aluminum nitride aluminum sulfide magnesium nitride

Tertiary Compounds ◦ contain polyatomic ions (see periodic table given in class) ◦ name the first element and then the polyatomic ion ◦ Use zero sum rule to write formula ◦ Try Table 2
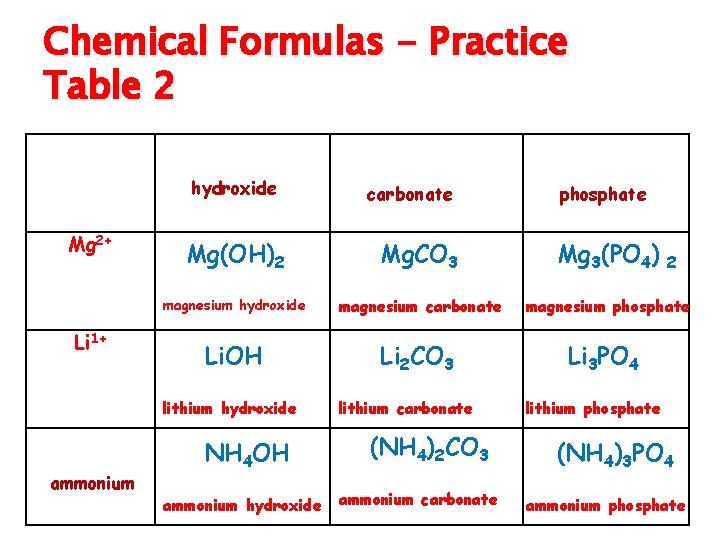
Chemical Formulas - Practice Table 2 OH 1 hydroxide Mg 2+ Li 1+ ammonium carbonate Mg(OH)2 Mg. CO 3 magnesium hydroxide magnesium carbonate Li. OH Li 2 CO 3 lithium hydroxide NH 41+ CO 32 - NH 4 OH ammonium hydroxide lithium carbonate (NH 4)2 CO 3 ammonium carbonate PO 43 phosphate Mg 3(PO 4) 2 magnesium phosphate Li 3 PO 4 lithium phosphate (NH 4)3 PO 4 ammonium phosphate
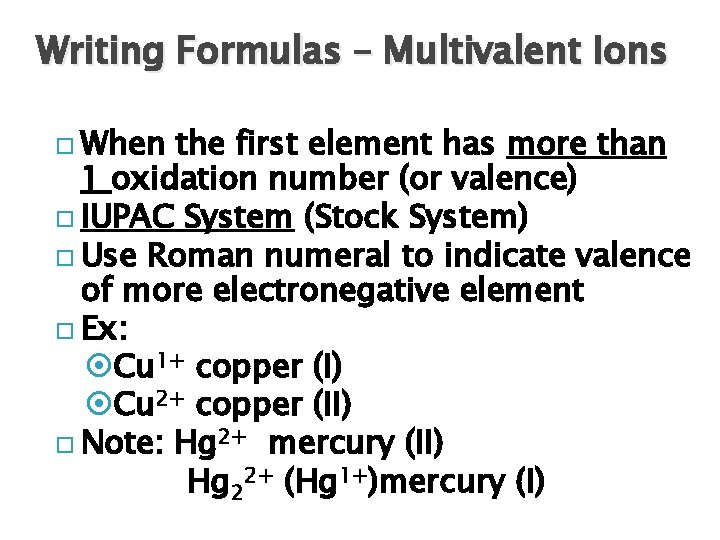
Writing Formulas – Multivalent Ions When the first element has more than 1 oxidation number (or valence) IUPAC System (Stock System) Use Roman numeral to indicate valence of more electronegative element Ex: Cu 1+ copper (I) Cu 2+ copper (II) Note: Hg 2+ mercury (II) Hg 22+ (Hg 1+)mercury (I)

Writing Formulas – Multivalent Ions Old system(Classical System)(ic/ous) Use suffix “ic” for highest valence and “ous” for lowest Use classical name of element (see table provided on next slide)
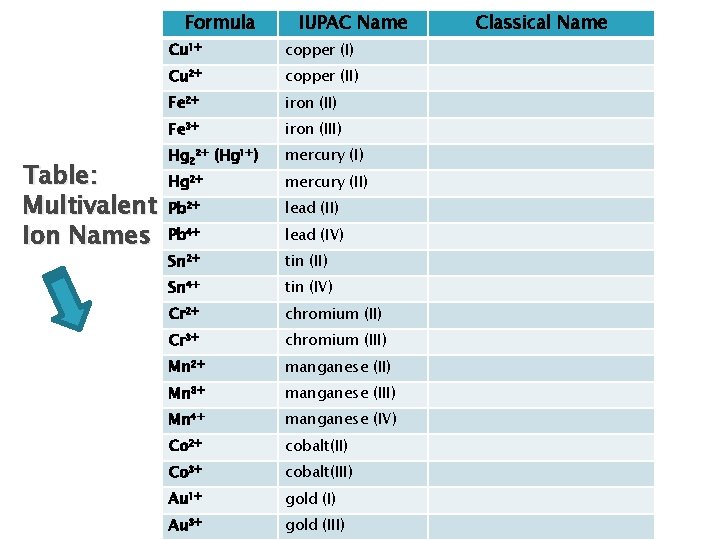
Formula Table: Multivalent Ion Names IUPAC Name Cu 1+ copper (I) Cu 2+ copper (II) Fe 2+ iron (II) Fe 3+ iron (III) Hg 22+ (Hg 1+) mercury (I) Hg 2+ mercury (II) Pb 2+ lead (II) Pb 4+ lead (IV) Sn 2+ tin (II) Sn 4+ tin (IV) Cr 2+ chromium (II) Cr 3+ chromium (III) Mn 2+ manganese (II) Mn 3+ manganese (III) Mn 4+ manganese (IV) Co 2+ cobalt(II) Co 3+ cobalt(III) Au 1+ gold (I) Au 3+ gold (III) Classical Name
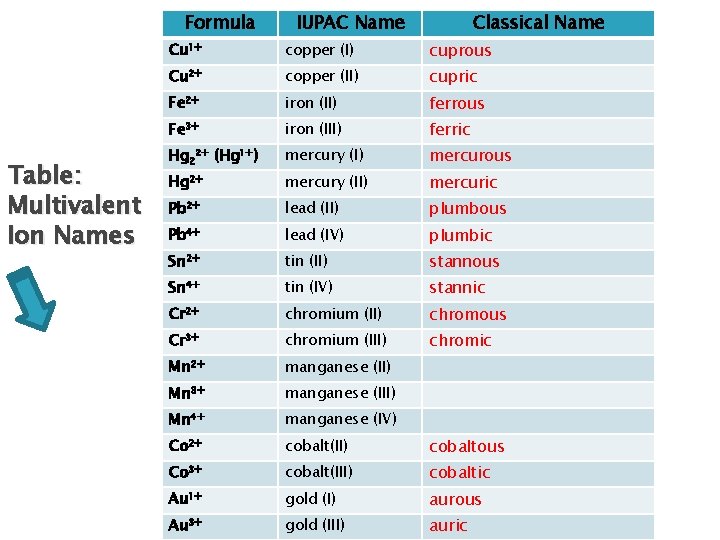
Formula Table: Multivalent Ion Names IUPAC Name Classical Name Cu 1+ copper (I) cuprous Cu 2+ copper (II) cupric Fe 2+ iron (II) ferrous Fe 3+ iron (III) ferric Hg 22+ (Hg 1+) mercury (I) mercurous Hg 2+ mercury (II) mercuric Pb 2+ lead (II) plumbous Pb 4+ lead (IV) plumbic Sn 2+ tin (II) stannous Sn 4+ tin (IV) stannic Cr 2+ chromium (II) chromous Cr 3+ chromium (III) chromic Mn 2+ manganese (II) Mn 3+ manganese (III) Mn 4+ manganese (IV) Co 2+ cobalt(II) cobaltous Co 3+ cobalt(III) cobaltic Au 1+ gold (I) aurous Au 3+ gold (III) auric
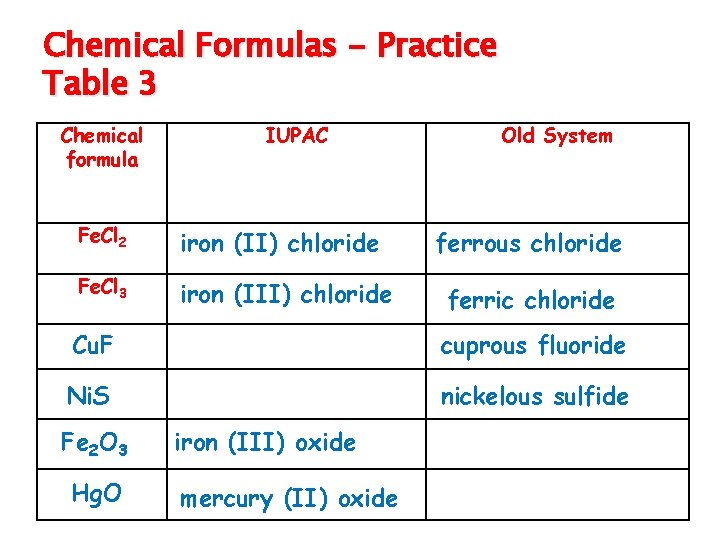
Chemical Formulas - Practice Table 3 Chemical formula IUPAC Old System Fe. Cl 2 iron (II) chloride ferrous chloride Fe. Cl 3 iron (III) chloride ferric chloride Cu. F Ni. S Fe 2 O 3 Hg. O copper (I) fluoride nickel (II) sulfide iron (III) oxide mercury (II) oxide cuprous fluoride nickelous sulfide ferric oxide mercuric oxide

Writing Formulas – Polyatomic Ions Some polyatomic ions contain the same types of atoms but different numbers of each type. (see periodic table given in class)
Writing Formulas – Polyatomic Ions For the related ions, only the “ate” ions need to be memorized, others may be found following this pattern: Step method:
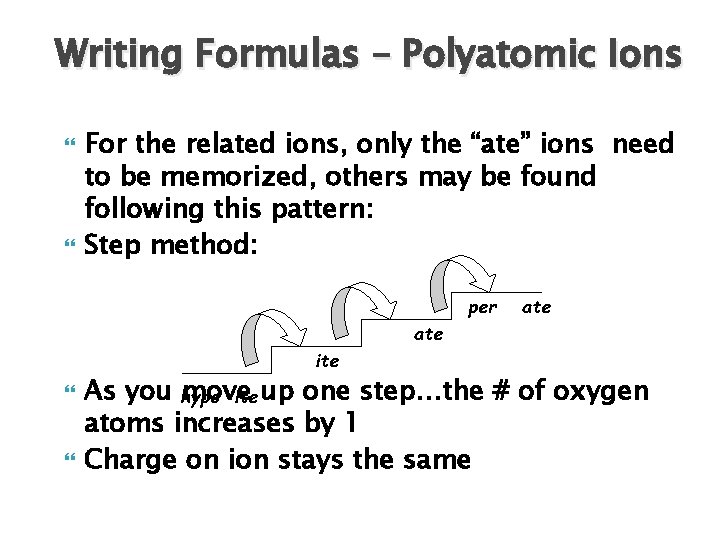
Writing Formulas – Polyatomic Ions For the related ions, only the “ate” ions need to be memorized, others may be found following this pattern: Step method: per ate ite As you hypo move ite up one step…the # of oxygen atoms increases by 1 Charge on ion stays the same

Oxy-Acid Radicals ◦ Try the oxy-acid radicals handout provided ◦ Note: Elements in the same group on the periodic table form the same polyatomic ion.

Chemical Bonding Chemical Formulas - Covalent
Covalent Compounds-Prefix Method When compounds contain 2 non-metals use the PREFIX METHOD for naming. Write the name of the 1 st element the first element is the least electronegative element (i. e. closest to the LHS of the periodic table) Write the name of the 2 nd element deleting the last few letters and adding “ide” Indicate the # atoms of each type with the appropriate prefix See p 105 Table 3. 8 (copy this) Note: “mono” is left out when there is a single atom of the first element.
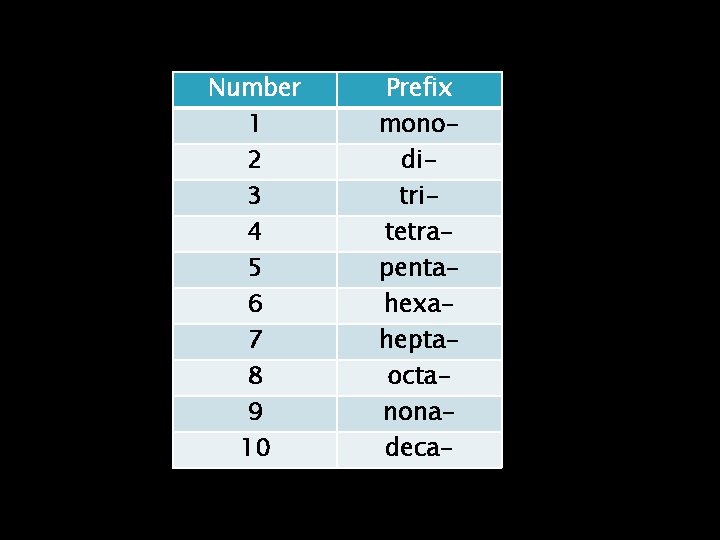
Number 1 2 3 4 5 6 7 8 9 10 Prefix monoditritetrapentahexaheptaoctanonadeca-
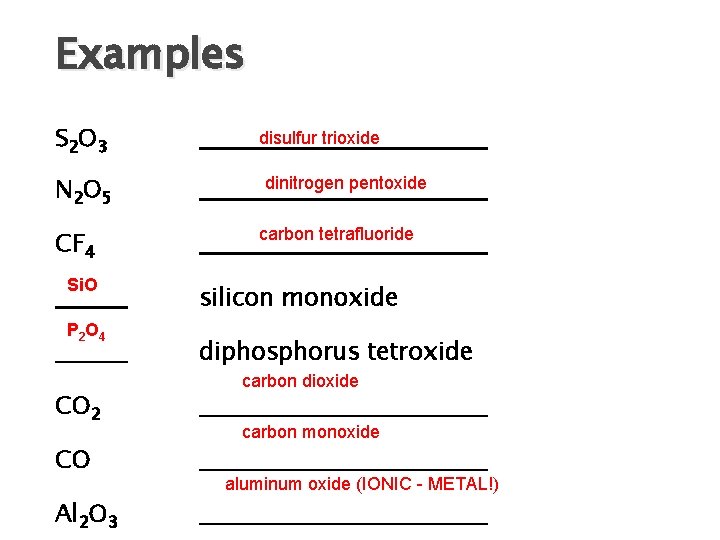
Examples S 2 O 3 N 2 O 5 CF 4 Si. O P 2 O 4 CO 2 CO Al 2 O 3 disulfur trioxide dinitrogen pentoxide carbon tetrafluoride silicon monoxide diphosphorus tetroxide carbon dioxide carbon monoxide aluminum oxide (IONIC - METAL!)
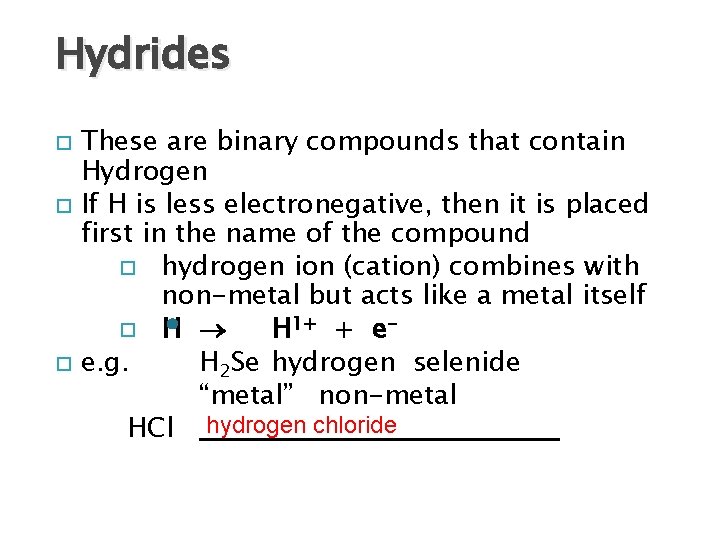
Hydrides These are binary compounds that contain Hydrogen If H is less electronegative, then it is placed first in the name of the compound hydrogen ion (cation) combines with non-metal but acts like a metal itself H H 1+ + ee. g. H 2 Se hydrogen selenide “metal” non-metal HCl hydrogen chloride

Hydrides Sometimes H can act like an anion (usually with a Group 1 metal)… so it is placed second in the name of the compound H + e- H 1 e. g. Na. H sodium hydride Ca. H 2 calcium hydride
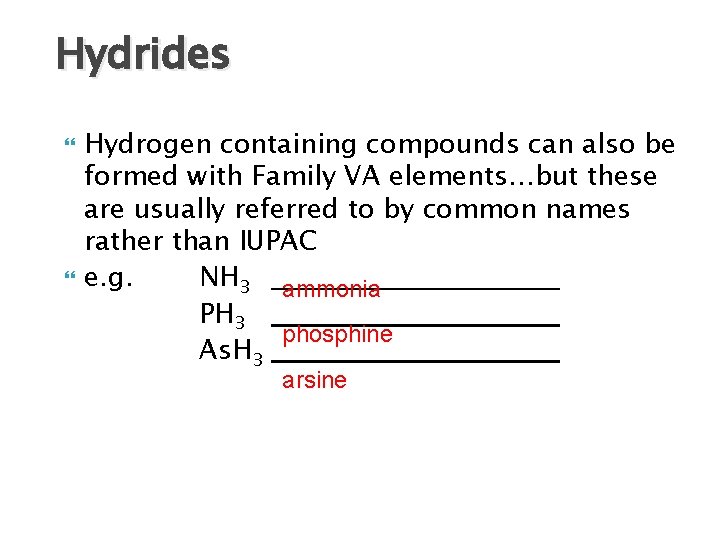
Hydrides Hydrogen containing compounds can also be formed with Family VA elements…but these are usually referred to by common names rather than IUPAC e. g. NH 3 ammonia PH 3 phosphine As. H 3 arsine
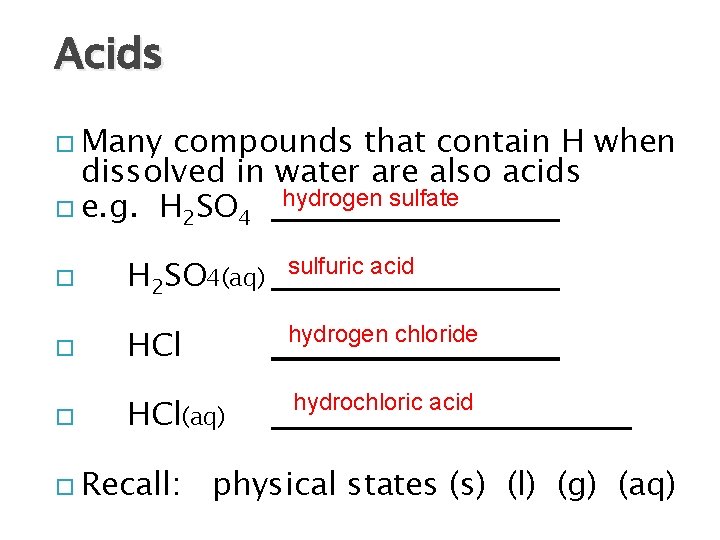
Acids Many compounds that contain H when dissolved in water are also acids e. g. H 2 SO 4 hydrogen sulfate H 2 SO 4(aq) sulfuric acid HCl hydrogen chloride HCl(aq) hydrochloric acid Recall: physical states (s) (l) (g) (aq)
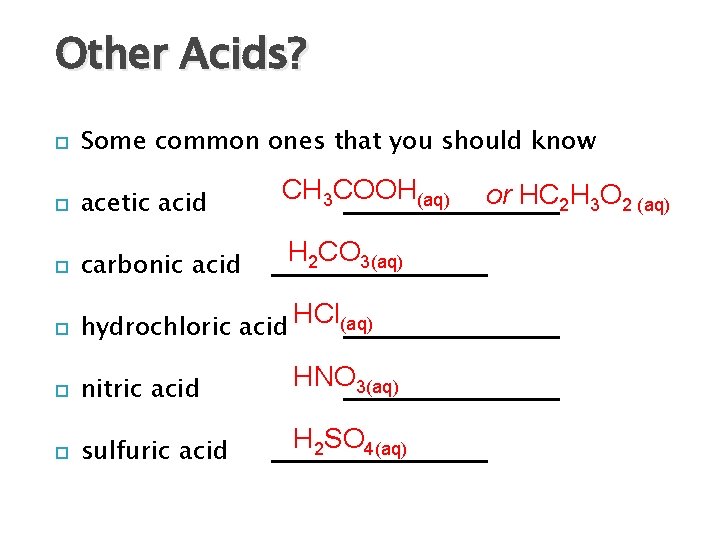
Other Acids? Some common ones that you should know acetic acid CH 3 COOH(aq) carbonic acid H 2 CO 3(aq) hydrochloric acid HCl(aq) nitric acid HNO 3(aq) sulfuric acid H 2 SO 4(aq) or HC 2 H 3 O 2 (aq)
- Slides: 50