Chemical Bonding Chemical bonds that hold atoms together

Chemical Bonding

Chemical bonds � ___________ that hold atoms together in compounds. The electrons involved in bonding are usually those in the ___________ (valence) shell. � Most elements in compounds want to gain ___________ configuration. They will do so by either _________or _________ electrons (___________ compounds) or by _________ electrons (___________ compounds)
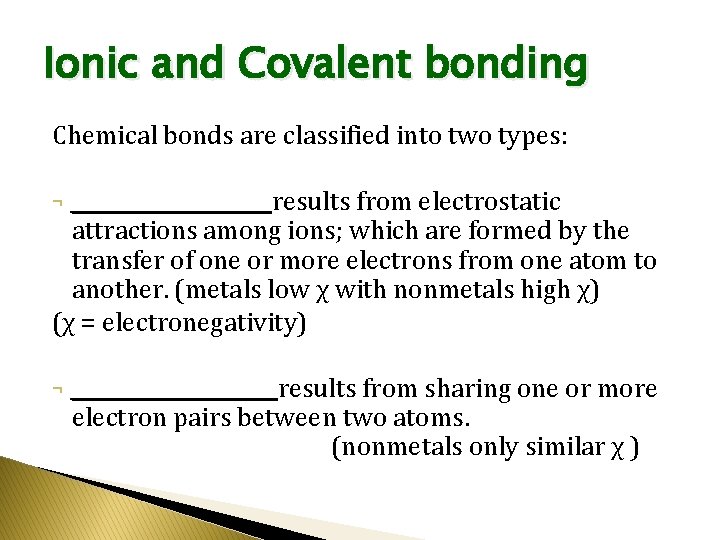
Ionic and Covalent bonding Chemical bonds are classified into two types: ___________results from electrostatic attractions among ions; which are formed by the transfer of one or more electrons from one atom to another. (metals low χ with nonmetals high χ) (χ = electronegativity) ¬ ¬ ___________ results from sharing one or more electron pairs between two atoms. (nonmetals only similar χ )
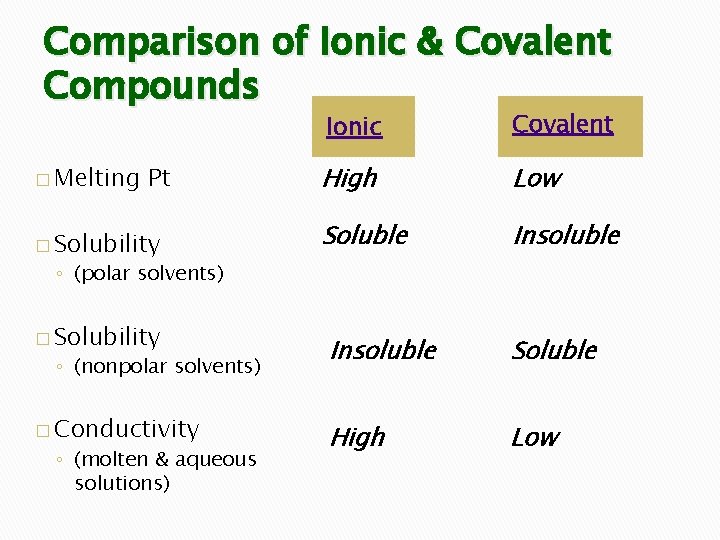
Comparison of Ionic & Covalent Compounds � Melting Pt � Solubility Ionic Covalent High Low Soluble Insoluble Soluble High Low ◦ (polar solvents) � Solubility ◦ (nonpolar solvents) � Conductivity ◦ (molten & aqueous solutions)
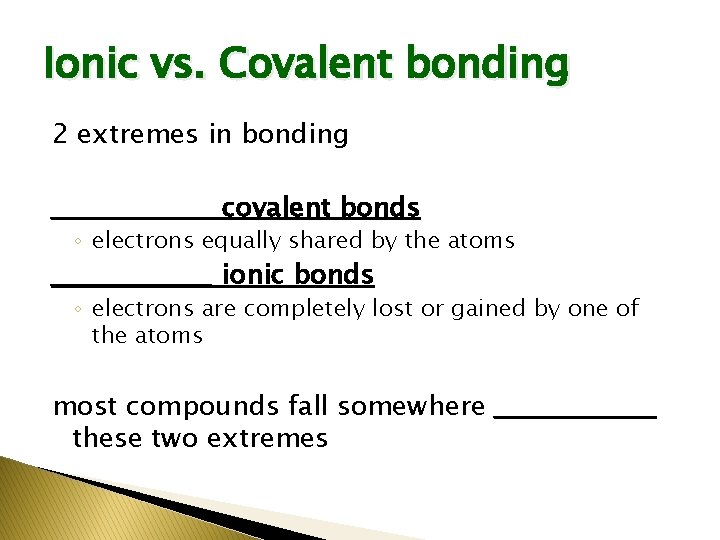
Ionic vs. Covalent bonding 2 extremes in bonding _________ covalent bonds ◦ electrons equally shared by the atoms _________ ionic bonds ◦ electrons are completely lost or gained by one of the atoms most compounds fall somewhere _________ these two extremes
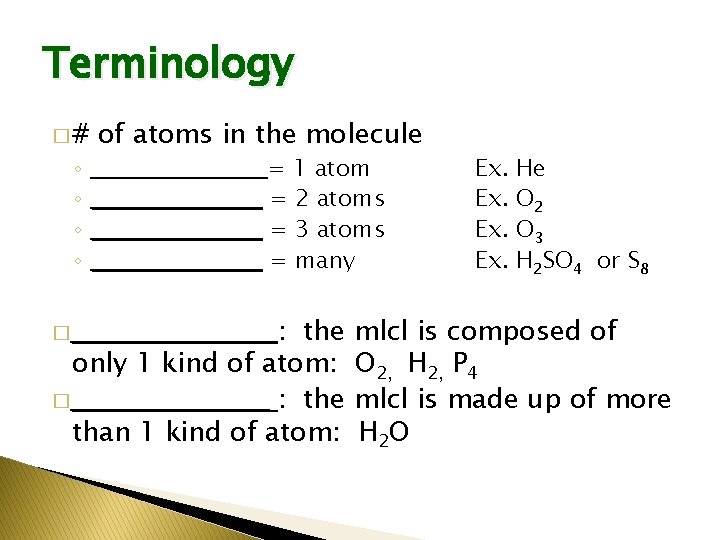
Terminology �# ◦ ◦ of atoms in the molecule ___________ = 1 atom ___________ = 2 atoms ___________ = 3 atoms ___________ = many : the only 1 kind of atom: � ___________ : the than 1 kind of atom: � ___________ Ex. Ex. He O 2 O 3 H 2 SO 4 or S 8 mlcl is composed of O 2, H 2, P 4 mlcl is made up of more H 2 O
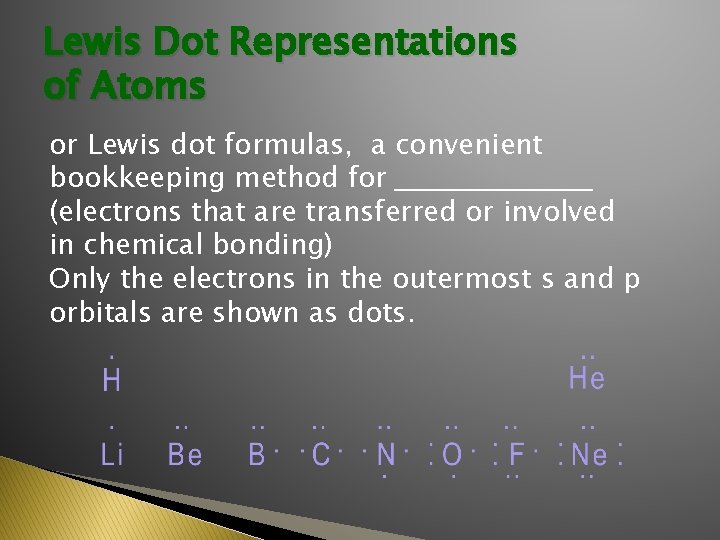
Lewis Dot Representations of Atoms or Lewis dot formulas, a convenient bookkeeping method for __________ (electrons that are transferred or involved in chemical bonding) Only the electrons in the outermost s and p orbitals are shown as dots.
elements in the same group have same Lewis dot structures For groups ___________, the group number equals the # of ___________ electrons Valence electrons determine the chemical and physical properties of the elements as well as the kinds of _________ they form.
Ionic Bonding metals react with nonmetals to form ionic compounds ___________ or positive (+) ions (metals) ◦ atoms have lost 1 or more electrons ___________ or negative (-) ions (nonmetals) ◦ atoms have gained 1 or more electrons
We can use Lewis formulas to represent the neutral atoms and the ions they form.
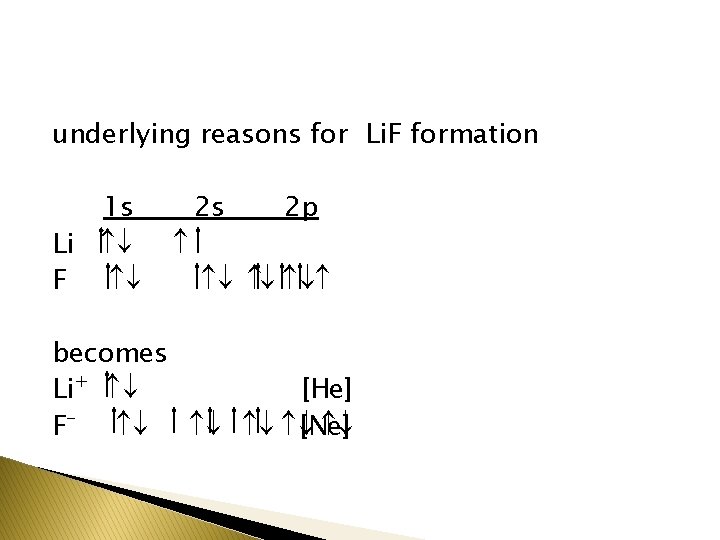
underlying reasons for Li. F formation 1 s Li ¯ F ¯ 2 s 2 p ¯ ¯ ¯ becomes Li+ ¯ [He] F- ¯ ¯ [Ne] ¯
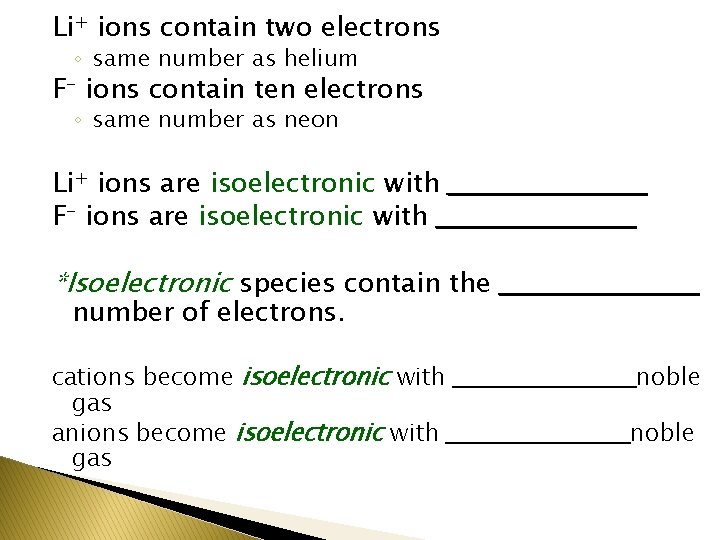
Li+ ions contain two electrons ◦ same number as helium F- ions contain ten electrons ◦ same number as neon Li+ ions are isoelectronic with ___________ F- ions are isoelectronic with ___________ *Isoelectronic species contain the ___________ number of electrons. cations become isoelectronic with ___________ noble gas anions become isoelectronic with ___________ noble gas
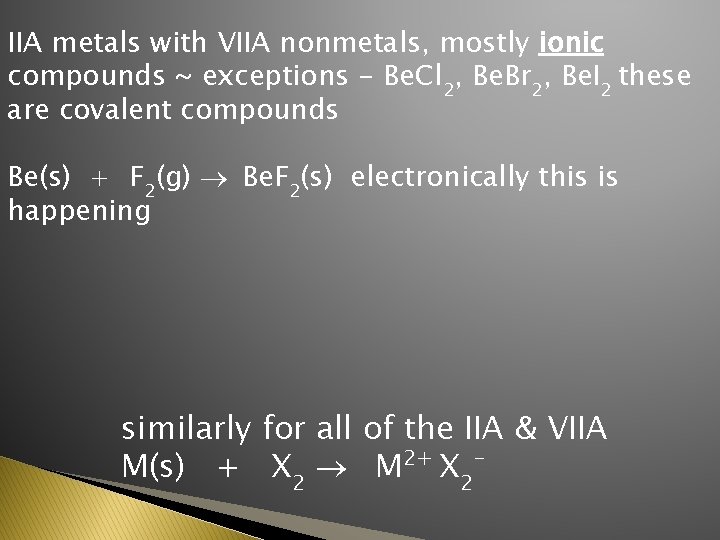
IIA metals with VIIA nonmetals, mostly ionic compounds ~ exceptions - Be. Cl 2, Be. Br 2, Be. I 2 these are covalent compounds Be(s) + F 2(g) ® Be. F 2(s) electronically this is happening similarly for all of the IIA & VIIA M(s) + X 2 ® M 2+ X 2 -
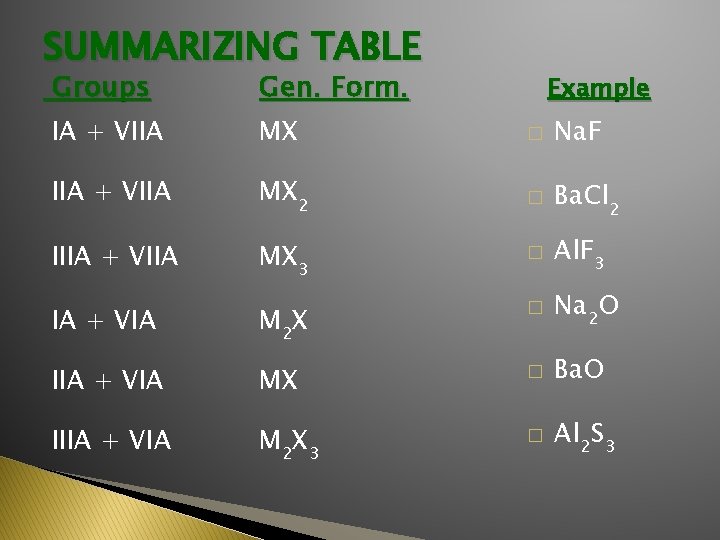
SUMMARIZING TABLE Groups Gen. Form. IA + VIIA MX � Na. F IIA + VIIA MX 2 � Ba. Cl 2 IIIA + VIIA MX 3 � Al. F 3 IA + VIA M 2 X � Na 2 O IIA + VIA MX � Ba. O IIIA + VIA M 2 X 3 � Al 2 S 3 Example
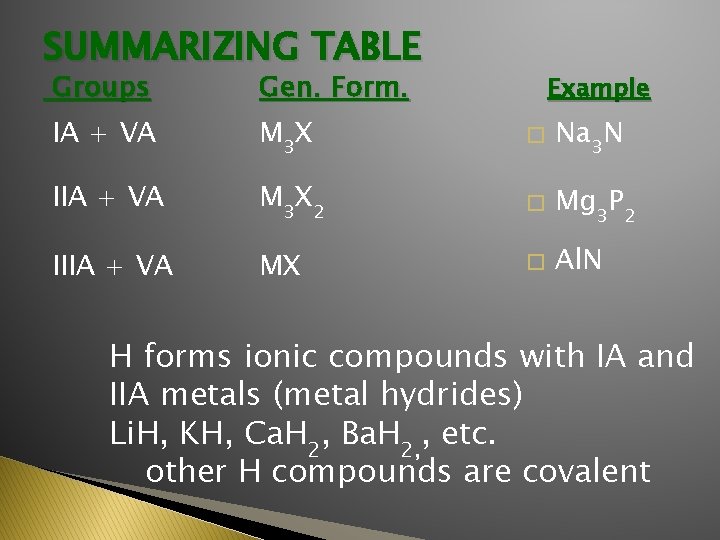
SUMMARIZING TABLE Groups Gen. Form. IA + VA M 3 X � Na 3 N IIA + VA M 3 X 2 � Mg 3 P 2 IIIA + VA MX � Al. N Example H forms ionic compounds with IA and IIA metals (metal hydrides) Li. H, KH, Ca. H 2, Ba. H 2, , etc. other H compounds are covalent

lattice energy ___________ - the energy needed to separate oppositely charged ions. It is the energy that converts an ionic solid into widely separated gas ions The ___________ the lattice energy, the ___________ the ionic bond. The stronger the ionic bond the ___________ in water at a given temperature, since the ions must _____________________ from one another and attach to water in order to dissolve.
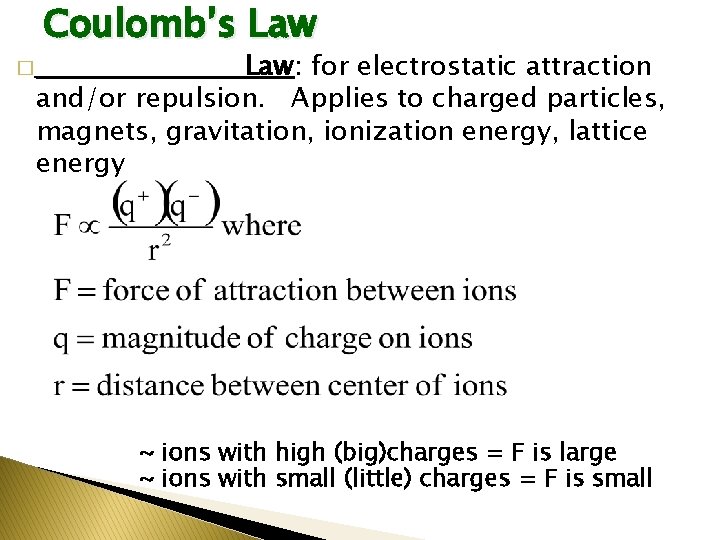
Coulomb’s Law: for electrostatic attraction and/or repulsion. Applies to charged particles, magnets, gravitation, ionization energy, lattice energy � ___________ ~ ions with high (big)charges = F is large ~ ions with small (little) charges = F is small
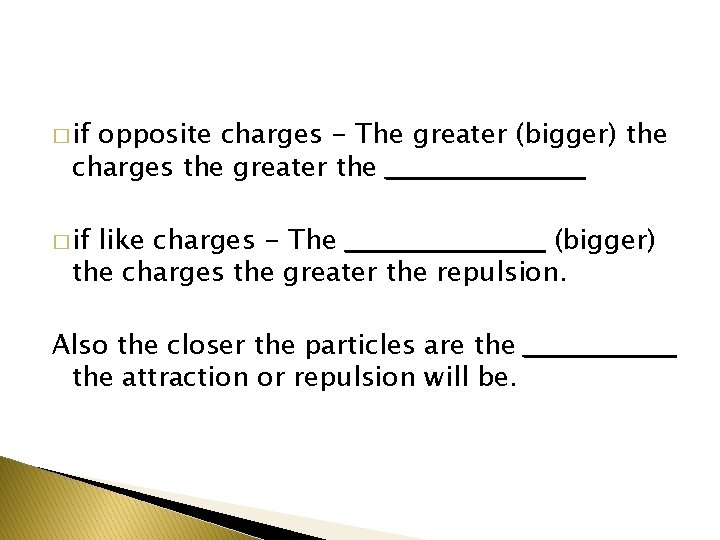
� if opposite charges - The greater (bigger) the charges the greater the ___________ � if like charges - The ___________ (bigger) the charges the greater the repulsion. Also the closer the particles are the ________ the attraction or repulsion will be.
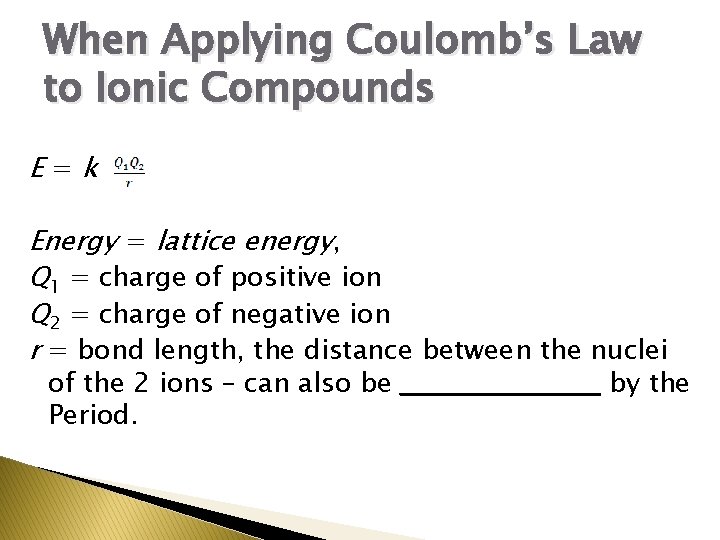
When Applying Coulomb’s Law to Ionic Compounds E=k Energy = lattice energy, Q 1 = charge of positive ion Q 2 = charge of negative ion r = bond length, the distance between the nuclei of the 2 ions – can also be ___________ by the Period.

r (Use the Period of the element that is ___________ with the ion! � For � Na is in Period 3 and has 3 occupied energy levels. Na+ has lost an electron. It has 10 electrons and is isoelectronic with Ne in Period 2. Na+ has only 2 occupied energy levels. )

arrange these compounds in order of increasing attractions among ions KCl, Al 2 O 3, Ca. O

1. Which has a stronger ionic bond, Na. Cl or KCl? Explain why. The lattice energy of ____ is stronger, so this is the stronger ionic bond. Both have an electron charge of -1 and an effective nuclear charge of 1. But the valence electrons of _____ are in the 3 rd energy level, leading to a shorter bond length (measured as distance between ionic nuclei) and a stronger ionic bond than ____.

2. Which has a stronger ionic bond, Na. Cl or Al. Cl 3? Explain why. The ____ has a stronger ionic bond. Both have the same -1 charge for the chloride ion, and both Na+ and Al+3 are isoelectronic with Ne and therefore have 2 occupied energy levels. But the higher positive charge of the ____ ion leads to a stronger lattice energy and a stronger ionic bond.
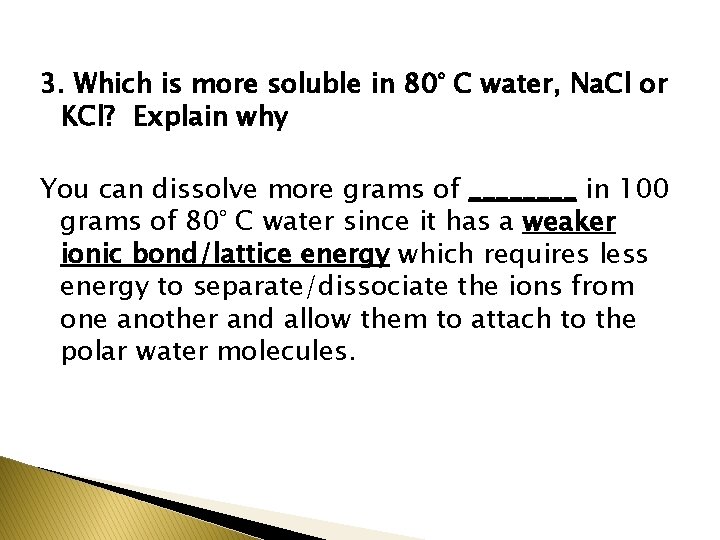
3. Which is more soluble in 80° C water, Na. Cl or KCl? Explain why You can dissolve more grams of ____ in 100 grams of 80° C water since it has a weaker ionic bond/lattice energy which requires less energy to separate/dissociate the ions from one another and allow them to attach to the polar water molecules.

4. Which is more soluble in 80° C water, Na. Cl or Al. Cl 3? Explain why ____ is more soluble in 80° C water since it has a weaker ionic bond/lattice energy which requires less energy to separate/dissociate the ions from one another and allow them to attach to the polar water molecules.

5. Why is Na 2 O considered soluble in water while Al 2 O 3 is not Q 2 for oxygen is the same for both compounds, a -2. Radius of oxygen is the same for both, and Na+ and Al+3 are isoelectronic. So the larger Q 1 charge of Al+3 makes the lattice energy of the ____ greater, and since the ions stay bonded to one another it will not dissociate and dissolve.

Structures of Ionic Compounds extended three dimensional arrays of oppositely charged ions ___________ points because coulomb force is strong
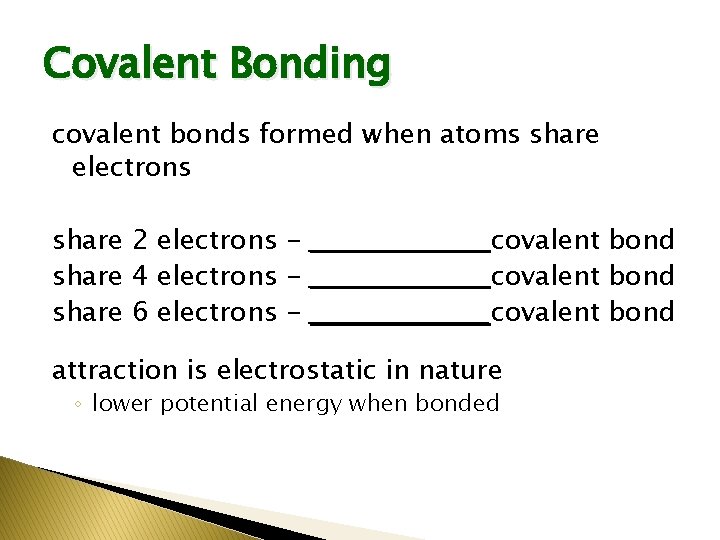
Covalent Bonding covalent bonds formed when atoms share electrons share 2 electrons - __________covalent bond share 4 electrons - __________covalent bond share 6 electrons - __________covalent bond attraction is electrostatic in nature ◦ lower potential energy when bonded
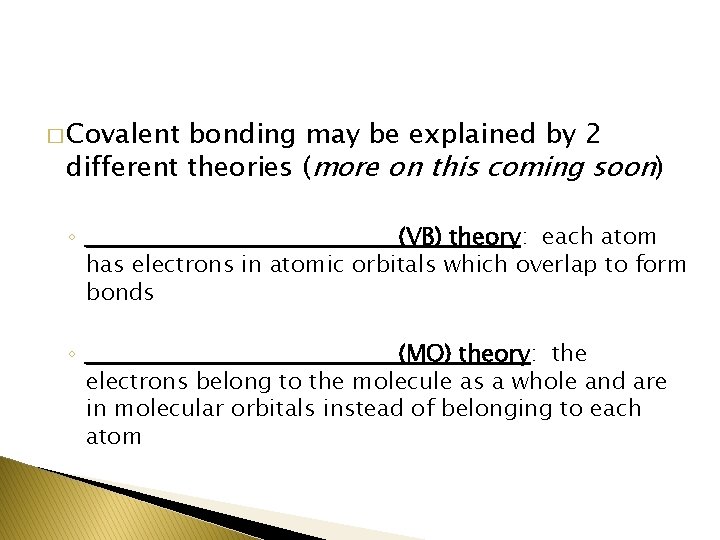
� Covalent bonding may be explained by 2 different theories (more on this coming soon) ◦ ___________________(VB) theory: each atom has electrons in atomic orbitals which overlap to form bonds ◦ ___________________(MO) theory: the electrons belong to the molecule as a whole and are in molecular orbitals instead of belonging to each atom
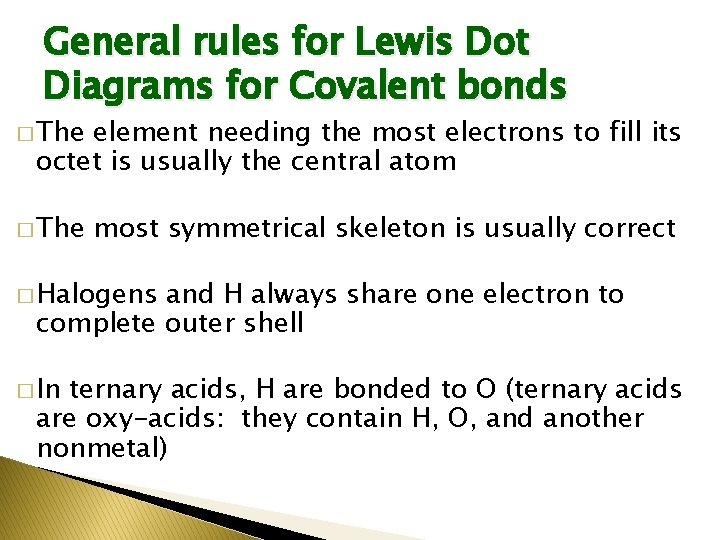
General rules for Lewis Dot Diagrams for Covalent bonds � The element needing the most electrons to fill its octet is usually the central atom � The most symmetrical skeleton is usually correct � Halogens and H always share one electron to complete outer shell � In ternary acids, H are bonded to O (ternary acids are oxy-acids: they contain H, O, and another nonmetal)
� Carbon always obeys the octet rule � Carbon rarely has lone pairs of electrons. Exception: If it’s at the end of a molecule or ion. Ex. CN- , CO, CNO � When forming multiple bonds between atoms, both atoms donate the same number of electrons
� Oxygen atoms normally bond to other nonmetals, not to each other � Oxygen can do several things depending on the mlcl. ◦ Single bond by sharing an electron ◦ Single bond by accepting 2 electrons from another atom and not sharing at all ◦ Double bonds by sharing 2 of its electrons
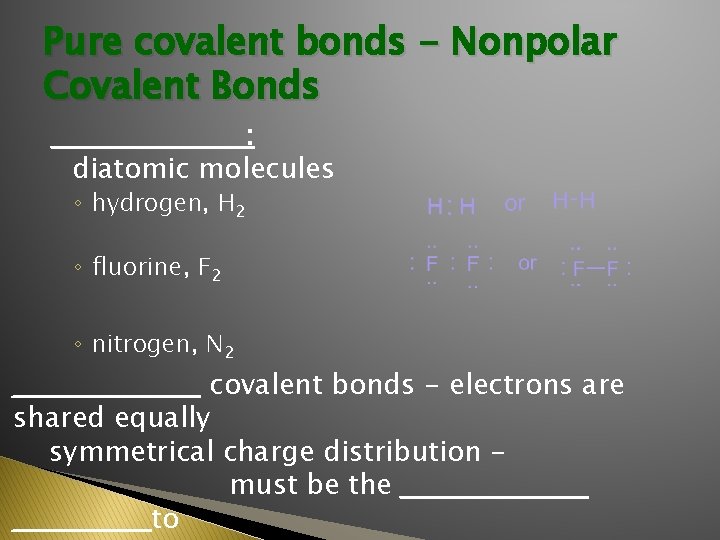
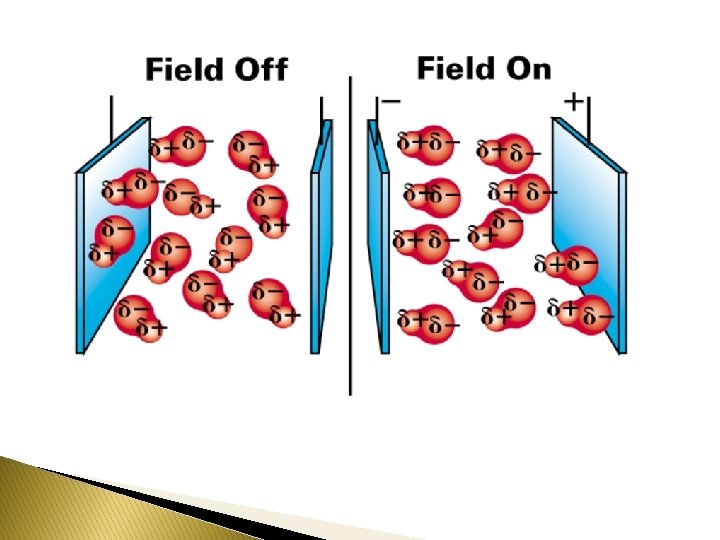
Pure covalent bonds - Nonpolar Covalent Bonds __________ : diatomic molecules ◦ hydrogen, H 2 ◦ fluorine, F 2 ◦ nitrogen, N 2 __________ covalent bonds - electrons are shared equally symmetrical charge distribution must be the __________to
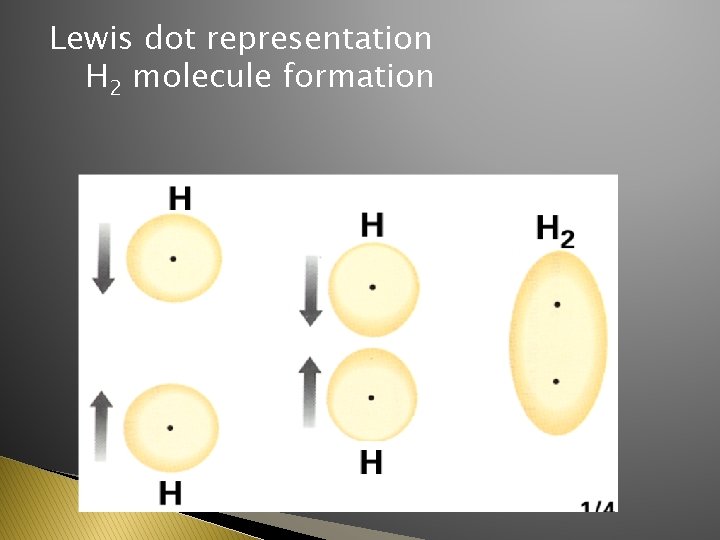
Lewis dot representation H 2 molecule formation

Polar Covalent bonds - Unequal sharing of electrons _______________: diatomic molecules hydrogen halides ◦ hydrogen fluoride, HF ◦ hydrogen chloride, HCl ◦ hydrogen bromide, HBr
__________ bonds - unequally shared electrons • __________ charge distribution • different ___________________ Some bonds are __________, Ex. HF
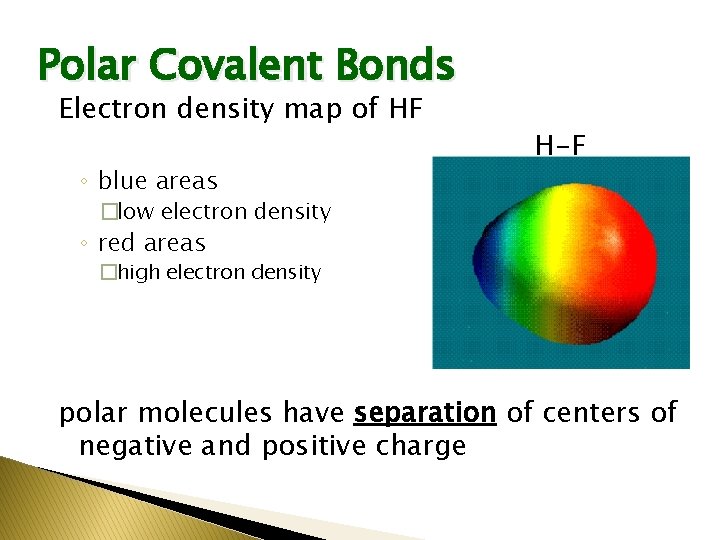
Polar Covalent Bonds Electron density map of HF ◦ blue areas H-F �low electron density ◦ red areas �high electron density polar molecules have separation of centers of negative and positive charge

Some bonds are only slightly polar, ex. HI
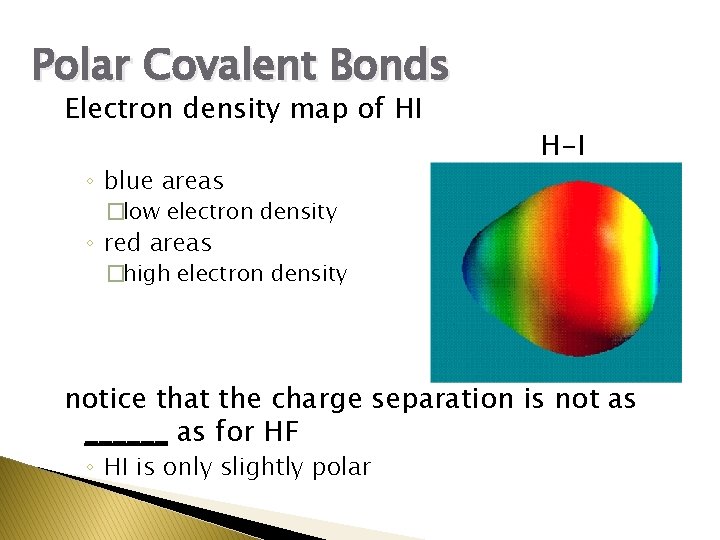
Polar Covalent Bonds Electron density map of HI ◦ blue areas H-I �low electron density ◦ red areas �high electron density notice that the charge separation is not as ______ as for HF ◦ HI is only slightly polar
The Octet Rule ___________________ elements achieve noble gas configurations in most of their compounds. Lewis dot formulas are based on the __________. �H needs two electrons to have Helium's noble gas configuration, everything else wants 8

Lewis Dot Formulas for Molecules and Polyatomic Ions water, H 2 O
Lewis Dot Formulas for Molecules and Polyatomic Ions ammonia molecule , NH 3

Lewis Dot Formulas for Molecules and Polyatomic Ions ammonium ion , NH 4+
Lewis Dot Formulas for Molecules and Polyatomic Ions hydrogen cyanide, HCN
Lewis Dot Formulas for Molecules and Polyatomic Ions sulfite ion, SO 32 -
Resonance � Two or more Lewis dot diagrams are needed to describe the bonding in a molecule or ion. � LDD for sulfur trioxide, SO 3

Resonance three possible structures for SO 3 invoke resonance ◦ Double-headed arrows are used to indicate resonance formulas.
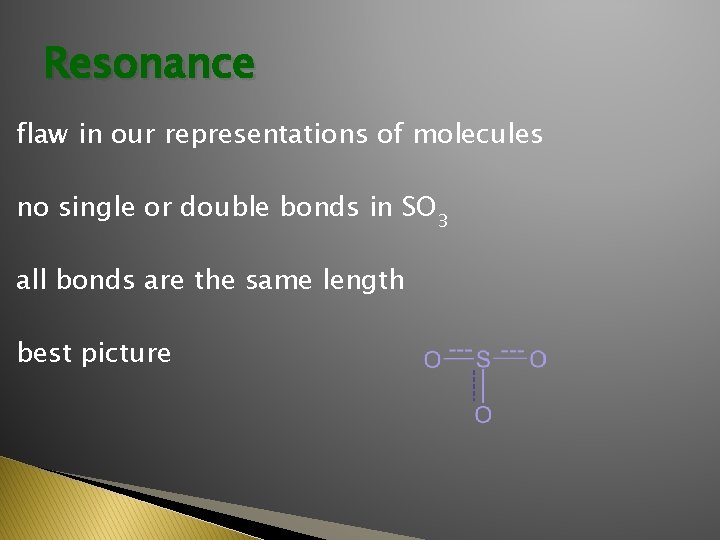
Resonance flaw in our representations of molecules no single or double bonds in SO 3 all bonds are the same length best picture

More Resonance structures � Carbonate, CO 3 -2

More Resonance structures � Nitrate, NO 3 -1

More Resonance structures � Nitrite, NO 2 -1
List of common Resonance structures � Sulfite ion, SO 32� Sulfur trioxide, SO 3 � Carbonate ion, CO 32� Nitrate ion, NO 31� Nitrite ion, NO 21� *Sulfate ion, SO 42� *Sulfur dioxide, SO 2 � *Ozone, O 3 � *Benzene, C 6 H 6
Formal Charges � The concept of formal charges helps us choose the correct Lewis structure for a molecule. If a ___________________ has a high formal charge it’s not a very good one. A formal charge is assigned to each element in a compound � Formal Or charge = group # - (e- you can assign to that atom) F. C. = (valence e- ) – (# of bonds + # of unshared e- )

� Let’s assign formal charges for the elements in the L. D. D. from water, H 2 O to sulfite ion, SO 32 -
Sigma and Pi bonds ___________________(σ) : result of headon (end to end overlap, there is a free rotation around σ bonds. ___________________(π) : result of sideon overlap of p orbitals. There is no free rotation around a π bond. The side –on overlap locks the molecule into place. All ________ bonds are sigma bonds: 1σ bond All ________ bonds: 1 σ bond, 1 π bond All ________ bonds: 1 σ bond, 2 π bonds
Limitations of the Octet Rule for Lewis Formulas ¹ º species in which the central element must have a share of more or less than 8 valence electrons to accommodate all substituents compounds of the d- and f-transition metals In cases where the octet rule does not apply, the elements attached to the central atom nearly always attain noble gas configurations. ◦ The central atom does not

Limitations of the Octet Rule for Lewis Formulas � Write LDD for BBr 3

Limitations of the Octet Rule for Lewis Formulas � Write LDD for As. F 5

Limitations of the Octet Rule for Lewis Formulas � Write LDD for Xe. F 4
- Slides: 60