Chemical Bonding Chemical Bonds are the forces of
Chemical Bonding

�Chemical Bonds are the forces of attraction holding atoms or ions together. �It is the valence electron of atom that forms the chemical bonds.

�There are two types of bonding: ◦ Ionic bonding � Electrons are transferred from one element to another ◦ Covalent bonding � Electrons are shared between elements

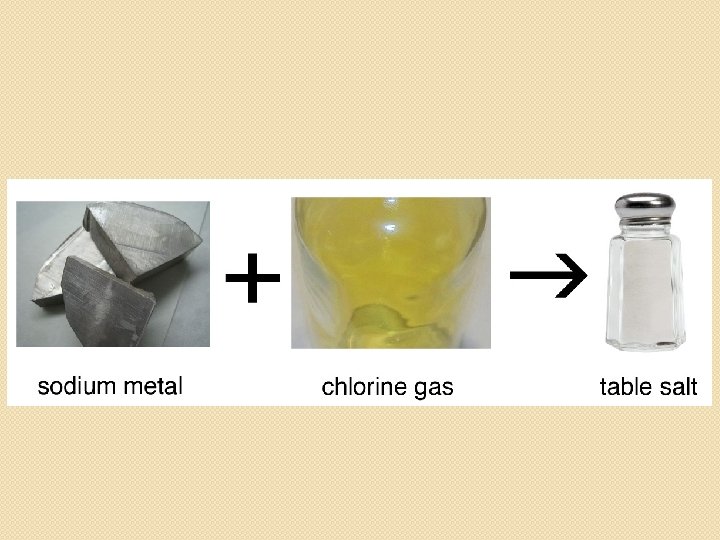
�Usually the formation of bonds produce compounds that are more stable than either of the two atoms on their own. Example: Sodium chloride, Na. Cl Na will burn when it touches water Cl 2 is a highly poisonous gas Na. Cl is harmless table salt, typically safe to eat

Ionic Bonding �Many elements form bonds that result in a full valence shell, so they have 8 valence electrons. (H and He will only have 2) �When this occurs, the ion is said to have a stable octet, this generalization is called the octet rule.

�Ionic compounds are formed when one or more valence electron is transferred from a metal atom to a non-metal atom. �This produces a positive metal ion (cation) and a negative non-metal ion (anion). �The two oppositely charged ions are attracted to each other by a force called an ionic bond.

�Example 1: Using Lewis structures show the formation of the bond between the following elements: �Sodium and chlorine �Magnesium and oxygen �Calcium and fluorine �Aluminum and chlorine
- Slides: 8