Chemical Bonding Chemical Bond nmutual electrical attraction between

Chemical Bonding
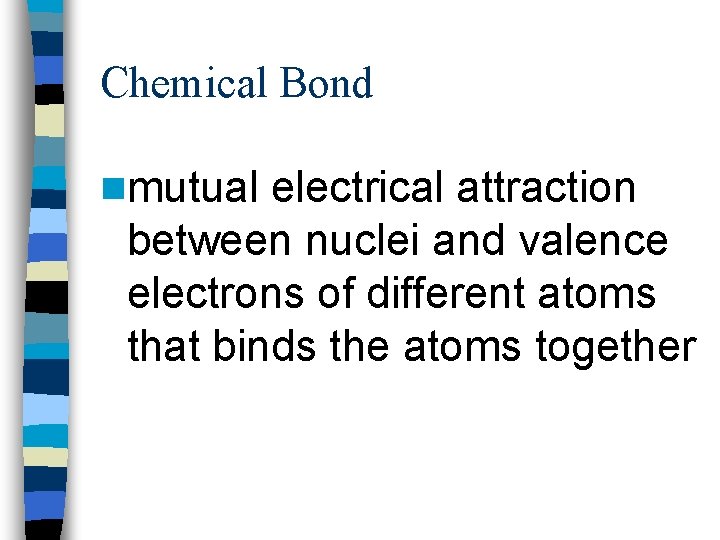
Chemical Bond nmutual electrical attraction between nuclei and valence electrons of different atoms that binds the atoms together
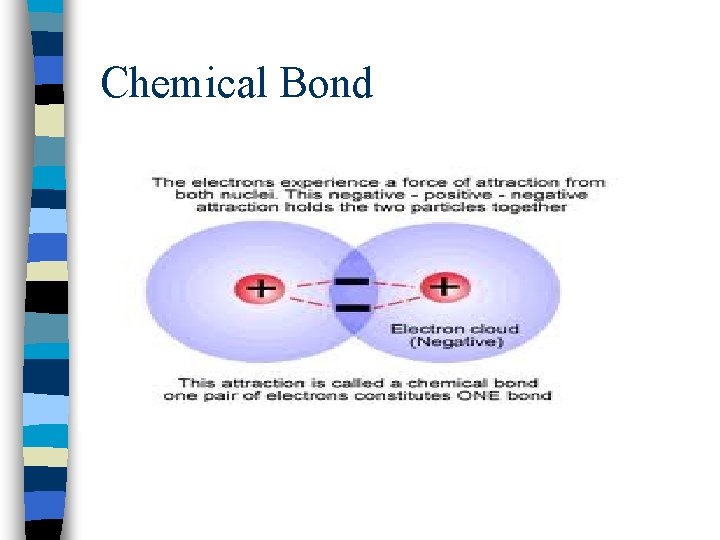
Chemical Bond

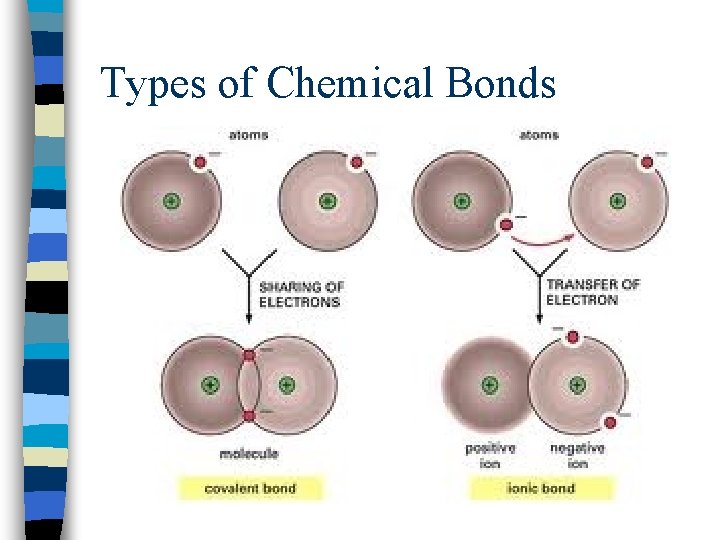
Types of Chemical Bonds n. Ionic bonding = results from the electrical attraction between cations and anions; one atom gives its electrons to another atom (metal and nonmetal)
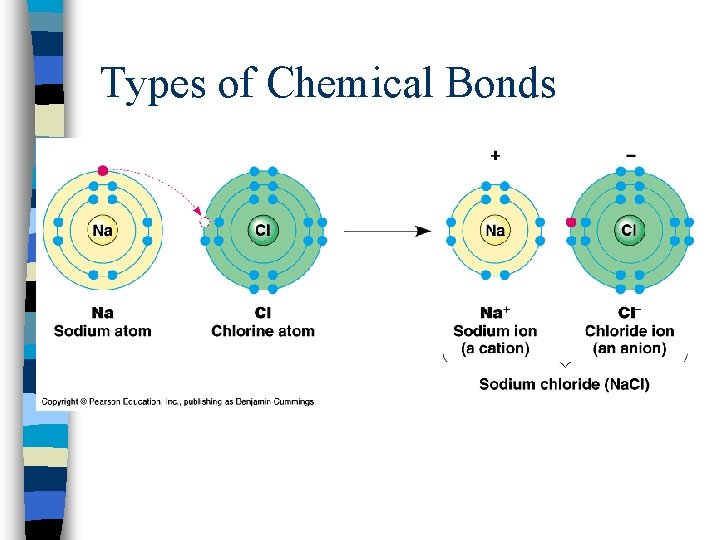
Types of Chemical Bonds

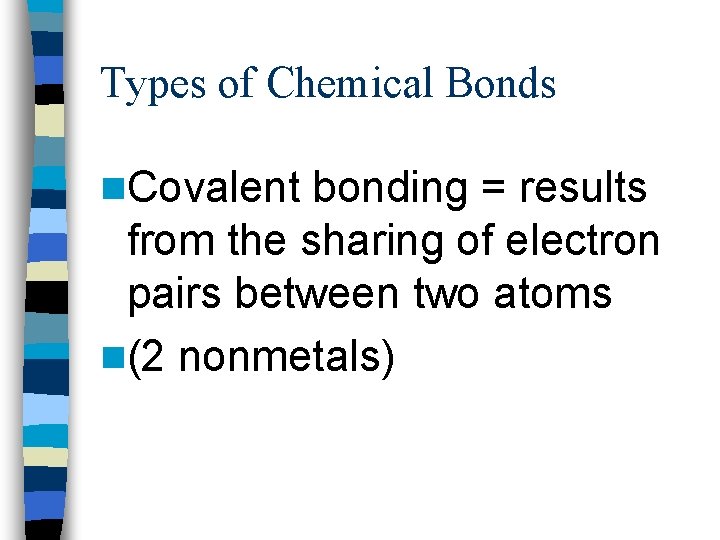
Types of Chemical Bonds n. Covalent bonding = results from the sharing of electron pairs between two atoms n(2 nonmetals)
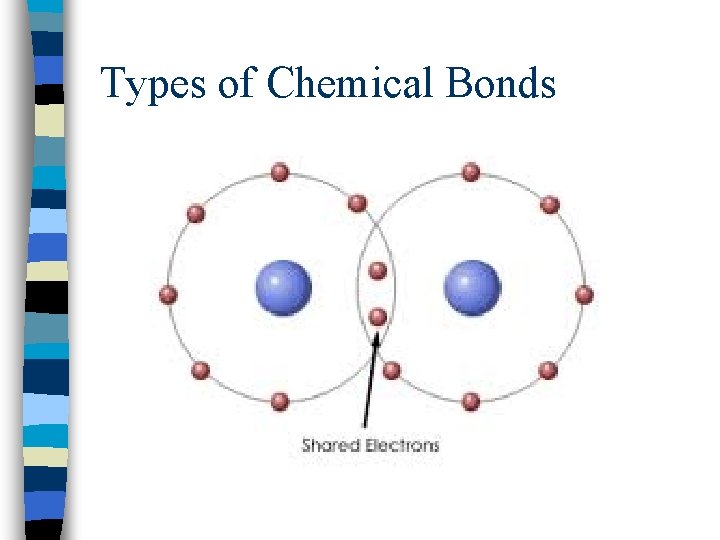
Types of Chemical Bonds

Types of Chemical Bonds n. Polar covalent bond = electrons are shared unequally by bonded atom resulting in an unbalanced charge distribution

Determining Bond Type
Types of Chemical Bonds n. Nonpolar covalent bond = electrons are shared equally by the bonded atoms, resulting in balanced distribution of charge
Types of Chemical Bonds
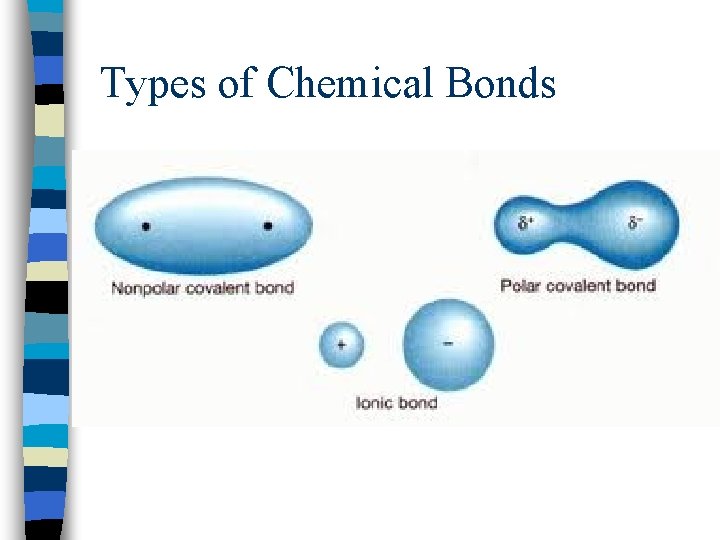
Types of Chemical Bonds
Determining Bond Type n Based on electronegativty
Determining Bond Type n Bond type can be estimated by calculating the difference in elements’ electronegativities n Ionic bonds: 1. 71 -3. 30 n Polar covalent: 0. 31 – 1. 70 n Nonpolar covalent: 0 -0. 30

Practice Problem n. Use the electronegativity differences to determine bonding in the following elements n. H and S , Ca and Cl, I and I
More Practice Problems n Use the electronegativity differences to determine bonding in the following compounds; then describe each bond type. n. Cl and Br, Cs and S, and P and O

Valence Electrons n the electrons in the highest occupied energy level of an element’s atoms n determine the properties of elements n equals the group number n used in bonding
Electron Dot Structures ndiagrams that show the valence electrons as dots placed around an element’s symbol
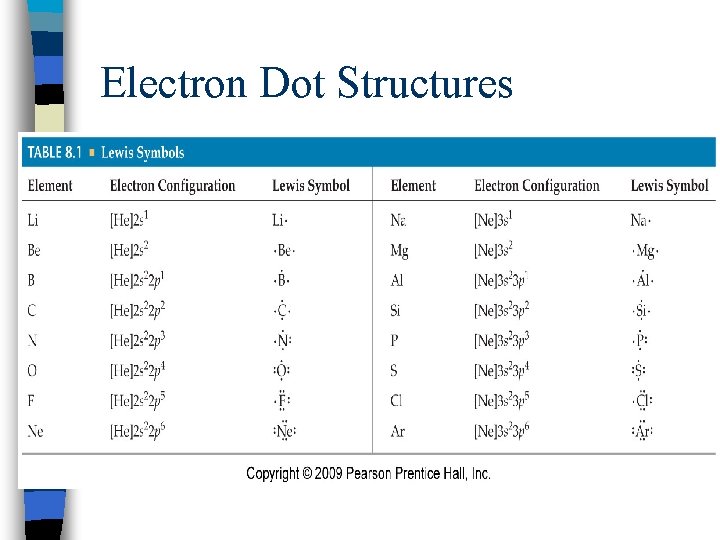
Electron Dot Structures
Octet Rule natoms bond achieve electron configurations of a noble gas, ns 2 np 6… a set of 8 electrons to become stable

Octet Rule n. Atoms of metallic elements tend to lose electrons to make a complete octet in the next lowest energy level
Octet Rule n. Atoms of nonmetallic elements gain or share electrons with another element to achieve a complete octet
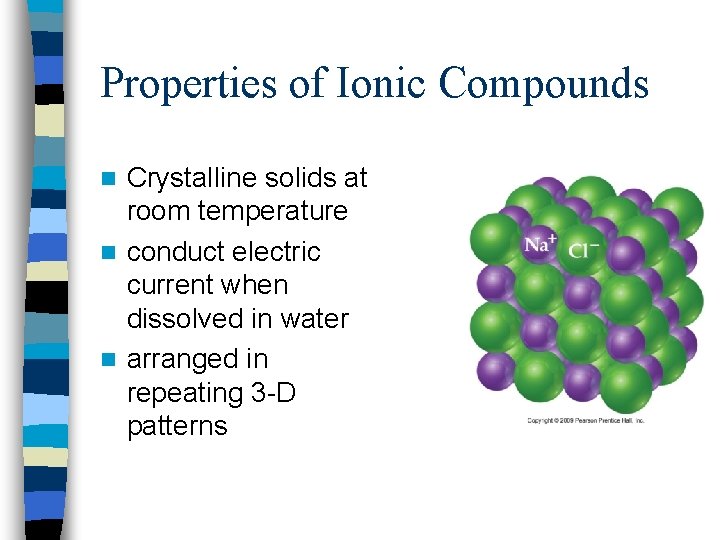
Properties of Ionic Compounds Crystalline solids at room temperature n conduct electric current when dissolved in water n arranged in repeating 3 -D patterns n
Formation of Ionic Compounds n 1. Draw Electron Dot Structure n 2. Show give/take of en 3. State what happened n 4. Write the formula
Formation of Ionic Compounds n. Show the formation of the bond between n. Na and Br n. Ca and Cl n. Al and S

Formation of Ionic Compounds n. Show the formation of the bond between n. Al + Br, K + O, Mg + N, Li + I, Ca +P
Covalent Bonding nelectrons are shared between nonmetals in groups 4 A, 5 A, 6 A, 7 A
Covalent Bonding n. Rules for drawing Lewis structures n 1. Add valence electrons for all atoms
Covalent Bonding n 2. Write symbols for atoms to show arrangement of atoms –C is always in the middle –H is always outside –Least electronegative atom is in the middle
Covalent Bonding n 3. Complete the octets of atoms bonded to central atom, except H n 4. Place leftover electrons on central atom n 5. If central atom is incomplete try a multiple bond
Single Covalent Bonding nbond in which two atoms share a pair of electrons n. Ex] H 2
Single Covalent Bonding n Structural Formulas - a shared pair of electrons are represented by a dash

Single Covalent Bonding n. Examples: n. Fluorine, Water n. Ammonia, n. Carbon tetrachloride n. Ge. F 4, PCl 3
Double and Triple Covalent Bonding n. Double bonds: share 2 pairs of electrons, (=) n. Triple bonds: share 3 pairs of electrons
Double and Triple Covalent Bonding n. Examples: n N 2 n. CO 2
Practice n Br 2 n Si. Cl 4 n HCl n O 2 n HCN n CO
Resonance Structures n structures that occur when it is possible to write 2 or more valid lewis structures that have the same number of electron pairs for a molecule or ion (O 3)
Polyatomic Ions ncovalently n. Examples: n. NH 4 + n. SO 3 2 - n. Cl. O 3 - bonded

Exceptions to the Octet Rule n. Some substances do not obey the octet rule and can have an incomplete or expanded octet n. BF 3, PCl 5, SF 6, Xe. F 4
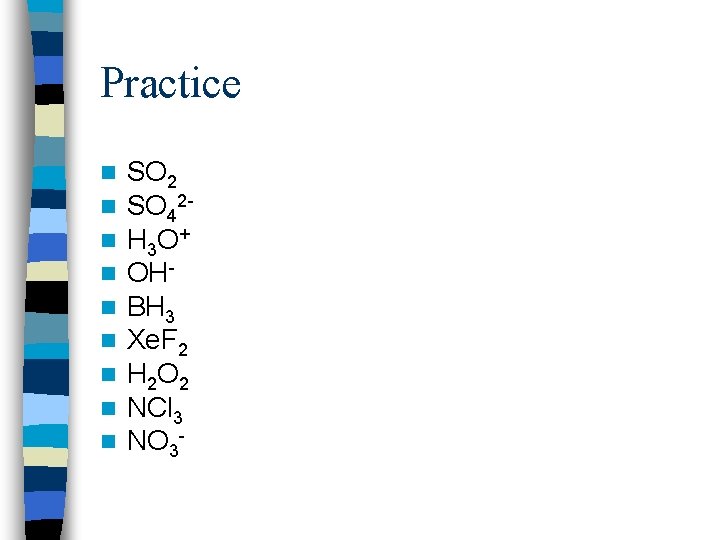
Practice n n n n n SO 2 SO 42 H 3 O + OHBH 3 Xe. F 2 H 2 O 2 NCl 3 NO 3 -
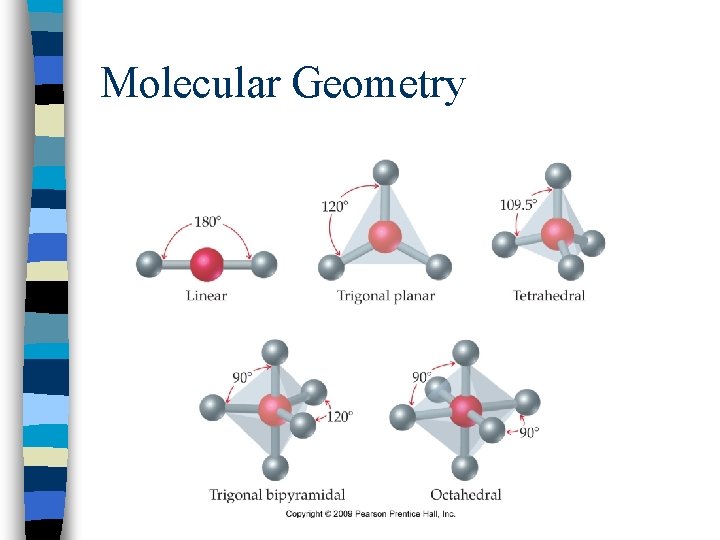
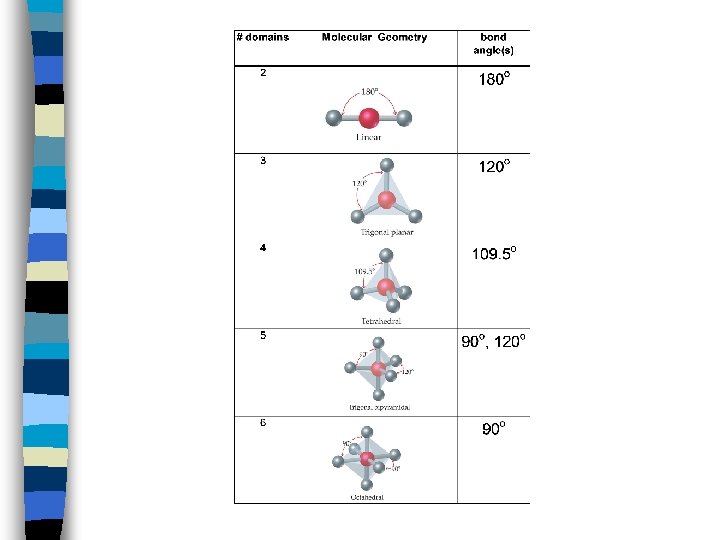
Molecular Geometry n. Shape of a molecule is determined by the bond angle which is based on the number of electron domains in the molecule
Molecular Geometry n. A pair of electrons, single, double, and triple bonds each consist of 1 electron domain
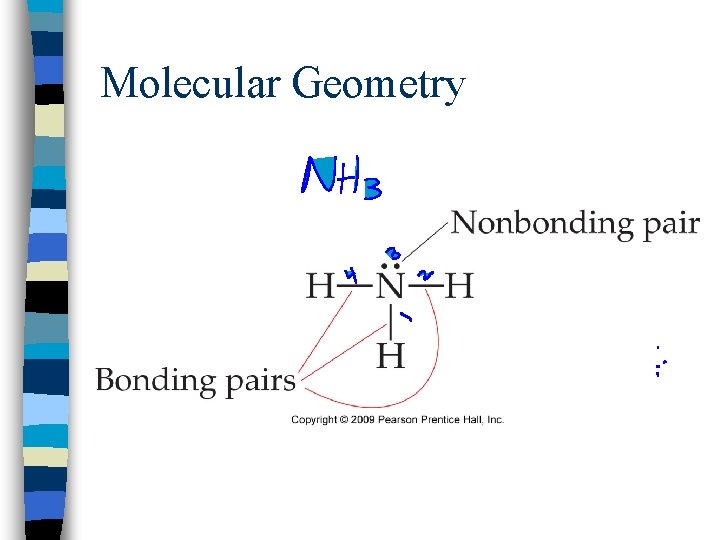
Molecular Geometry
Molecular Geometry n. To Predict Shapes: n 1. Draw Lewis structure and count e-d’s n 2. Predict bond angles and molecular geometry using table
Molecular Geometry
Molecular Geometry n Examples: n CO 2 n BF 3 n CBr 4 n PCl 5 n SF 6
Molecular Geometry n Practice: n Be. F 2 n BCl 3 n Si. H 4 n As. F 5 n NO 3 n Cl. O 4 - Predict Shape and Angle
Intramolecular vs. Intermolecular Intramolecular Forces – forces within molecules – chemical bonds n. Intermolecular Forces – forces between molecules – IMF’s n
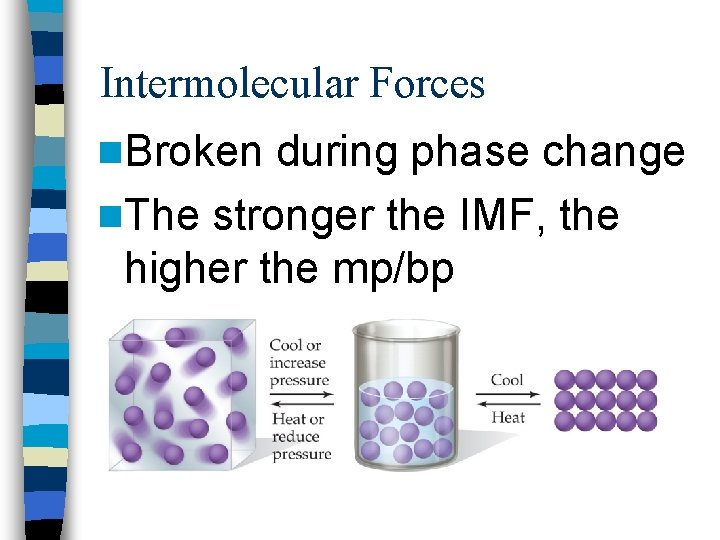
Intermolecular Forces n. Broken during phase change n. The stronger the IMF, the higher the mp/bp
Types of Intermolecular forces n hydrogen bonding: n attraction between the H atom in a molecule with H-F, H-O, or H-N and an unshared electron pair on N, F, or O
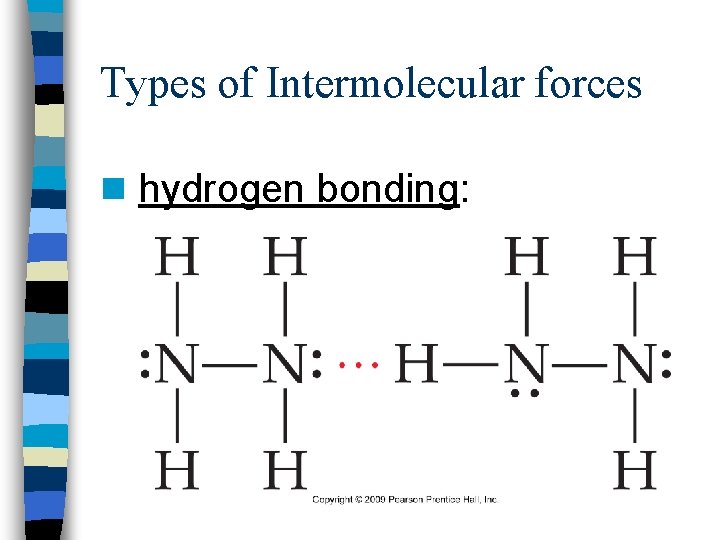
Types of Intermolecular forces n hydrogen bonding:

Properties of Water

Unique Properties of Water n 1. Hydrogen bonds cause density of solid H 2 O to be less than liquid H 2 O, so ice floats. n 2. Hydrogen bonds cause high surface tension in water.

Properties of Water n https: //www. youtube. com/watch ? v=Hm 52 rkh 68 JA
- Slides: 59