Chemical Bonding Chapter 8 Polarity and Dipole moments

Chemical Bonding Chapter 8 Polarity and Dipole moments
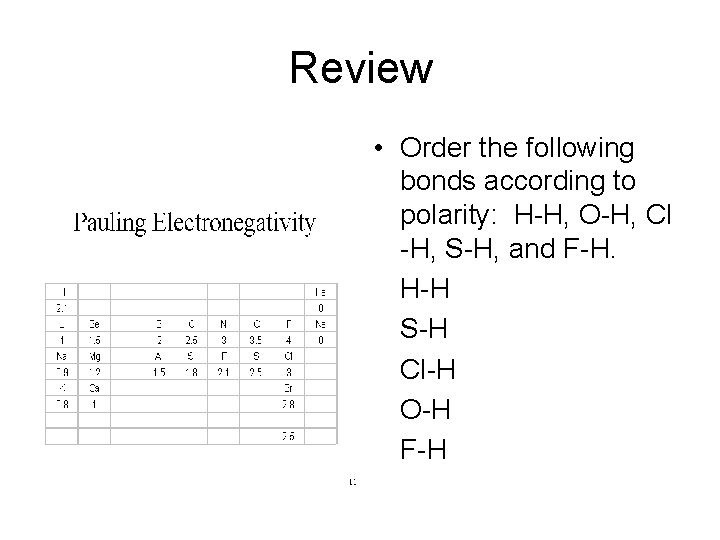
Review • Order the following bonds according to polarity: H-H, O-H, Cl -H, S-H, and F-H. • H-H • S-H • Cl-H • O-H • F-H
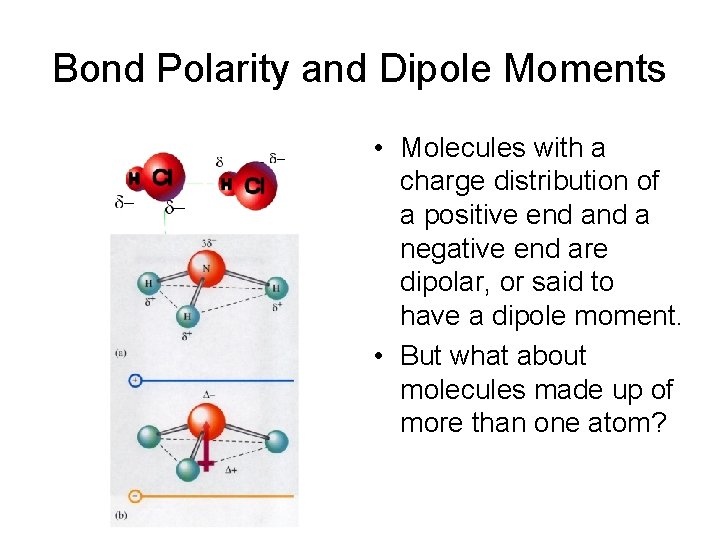
Bond Polarity and Dipole Moments • Molecules with a charge distribution of a positive end a negative end are dipolar, or said to have a dipole moment. • But what about molecules made up of more than one atom?
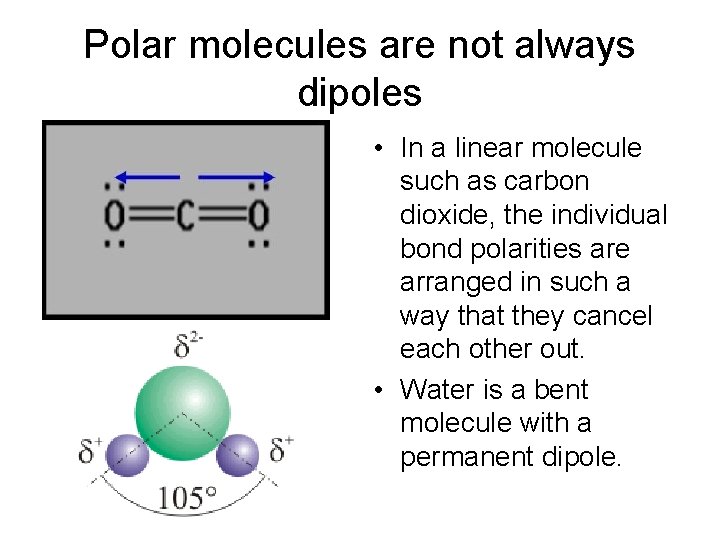
Polar molecules are not always dipoles • In a linear molecule such as carbon dioxide, the individual bond polarities are arranged in such a way that they cancel each other out. • Water is a bent molecule with a permanent dipole.
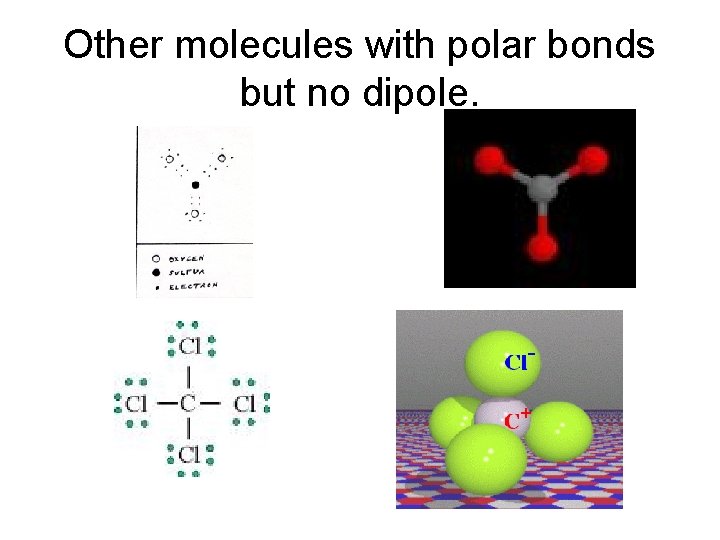
Other molecules with polar bonds but no dipole.
Electron Configurations • Electron arrangement helps us to understand configurations of compounds. • In stable compounds, virtually every atom has a noble gas type arrangement of electrons.

Generalizations of electrons in stable compounds • Two non-metals react to form covalent bonds in a way that completes the valence electron configuration of both atoms. • A metal and a non-metal react to form a binary ionic compound. The ions form to achieve the electron configuration of the nearest noble gas.
Predicting formation of ions • When discussing ionic compounds, scientists are generally referring to ions in their solid state, not gaseous state.
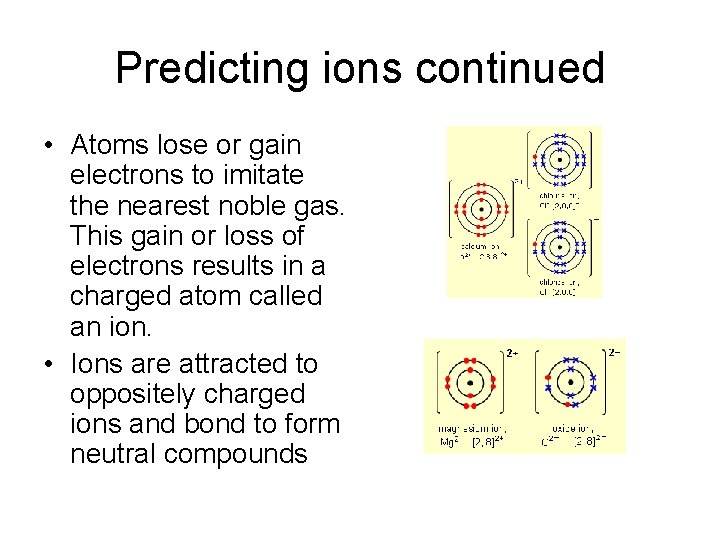
Predicting ions continued • Atoms lose or gain electrons to imitate the nearest noble gas. This gain or loss of electrons results in a charged atom called an ion. • Ions are attracted to oppositely charged ions and bond to form neutral compounds
Exceptions • • • Elements in Group 1 A lose an electron. Elements in Group 2 A lose 2 electrons. Elements in Group 7 A gain an electron. Elements in Group 6 A gain 2 electrons, etc. But…elements Sn may lose 2 or 4 electrons. • Pb 2+ or Pb 4+, Bi 3+ or Bi 5+, Cu 1+ or Cu 2+ and so on.
Size and Charge • Ion size is important in determining the structure and stability of ionic solids. • What determines the Size? • Look first at relative size of ion and its parent atom.
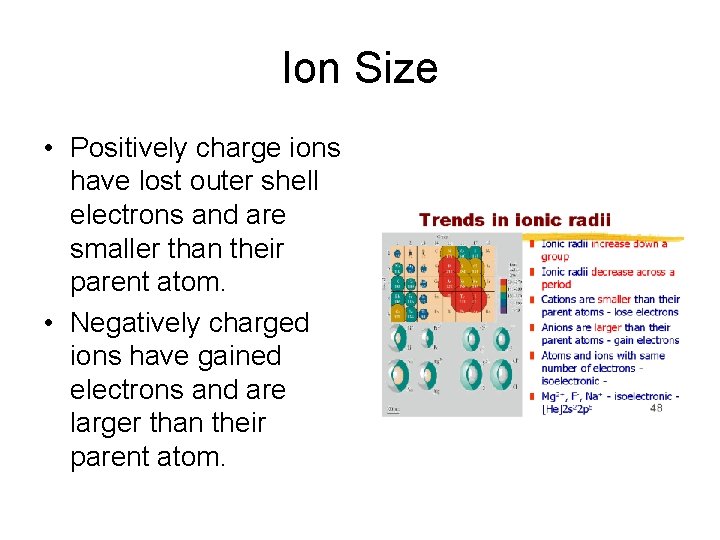
Ion Size • Positively charge ions have lost outer shell electrons and are smaller than their parent atom. • Negatively charged ions have gained electrons and are larger than their parent atom.
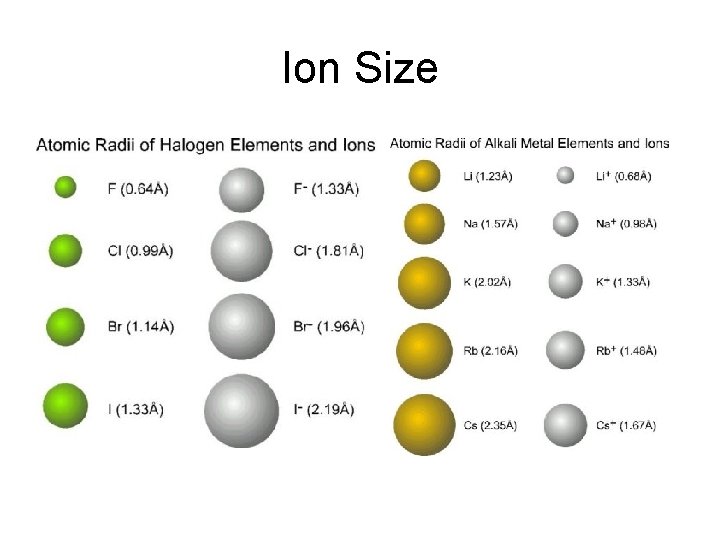
Ion Size
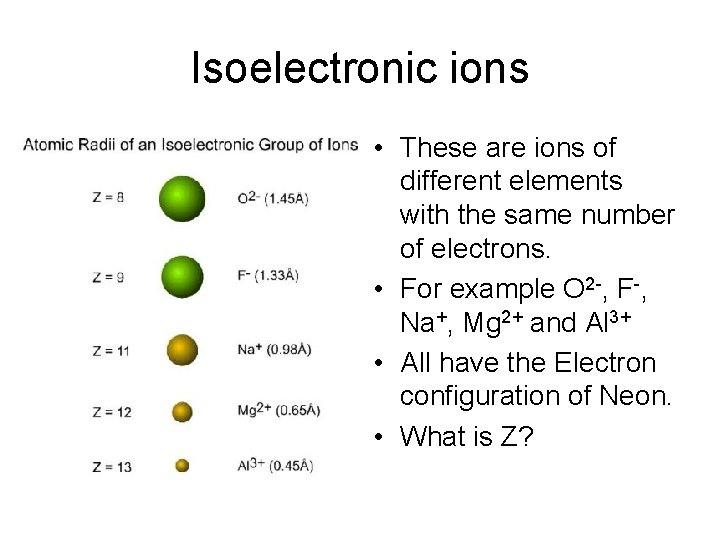
Isoelectronic ions • These are ions of different elements with the same number of electrons. • For example O 2 -, F-, Na+, Mg 2+ and Al 3+ • All have the Electron configuration of Neon. • What is Z?
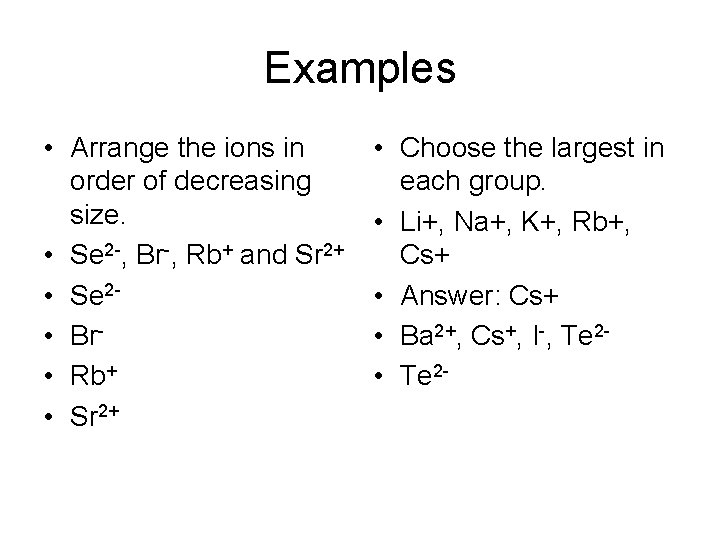
Examples • Arrange the ions in order of decreasing size. • Se 2 -, Br-, Rb+ and Sr 2+ • Se 2 • Br • Rb+ • Sr 2+ • Choose the largest in each group. • Li+, Na+, K+, Rb+, Cs+ • Answer: Cs+ • Ba 2+, Cs+, I-, Te 2 • Te 2 -
- Slides: 15