Chemical Bonding Chapter 8 AP Chemistry Types of

Chemical Bonding Chapter 8 AP Chemistry
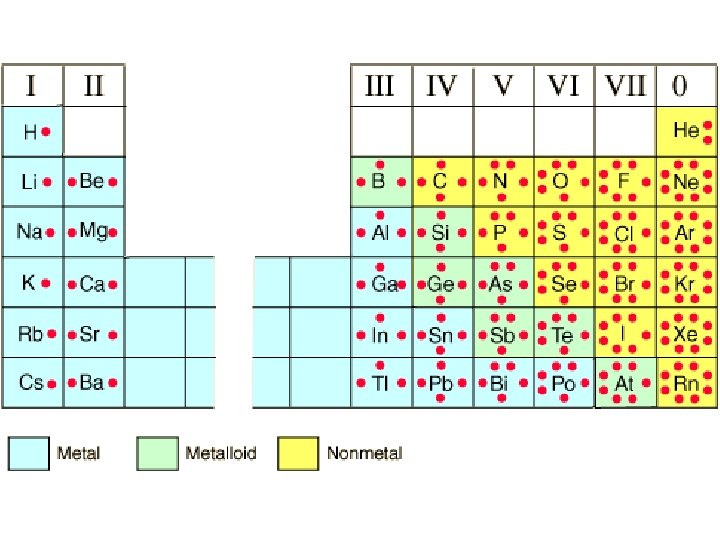
Types of Chemical Bonds • Ionic – electrons are transferred from a metal to a nonmetal • Covalent – electrons are shared between 2 nonmetals • Metallic – between metal atoms
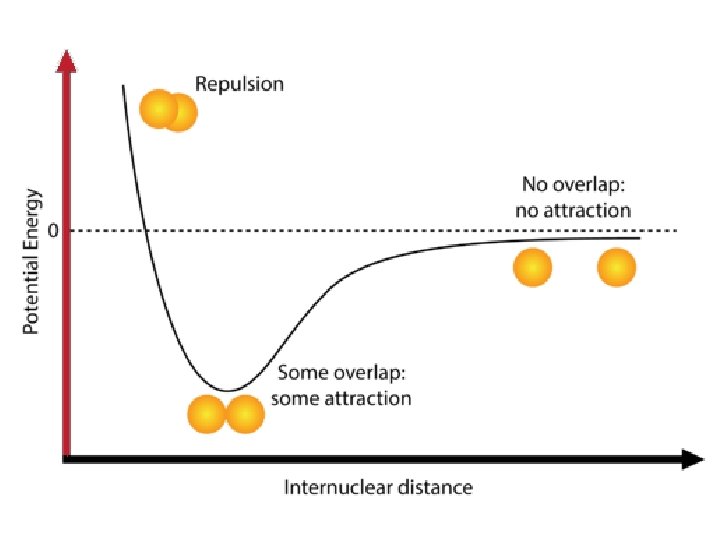
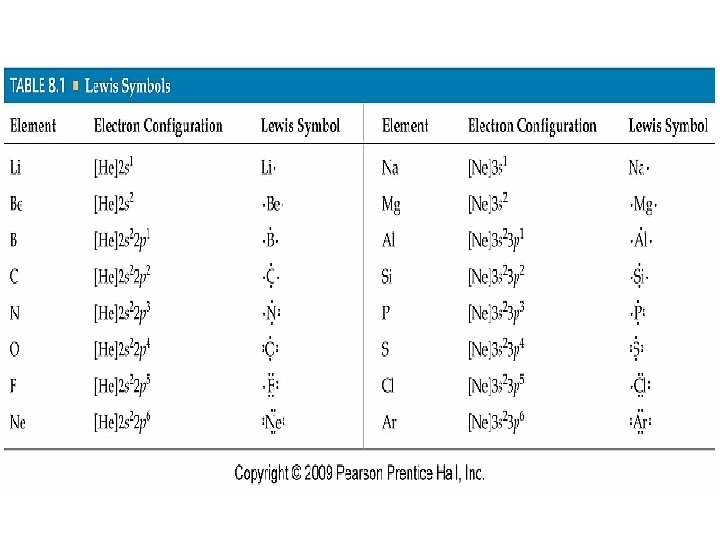
Lewis Symbols • Since bonding involves the element’s valence electrons, Lewis symbols are used. • Lewis symbols consist of the element symbol plus a dot for each valence electron. • Example: [Ne]3 s 23 p 4 (sulfur)
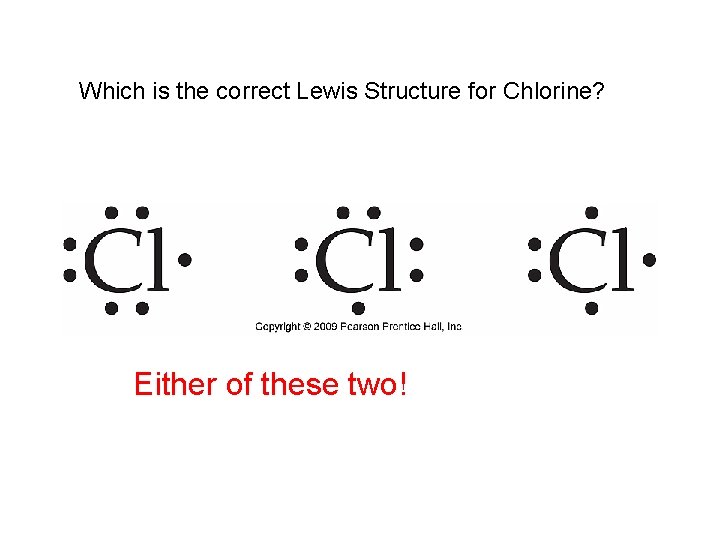
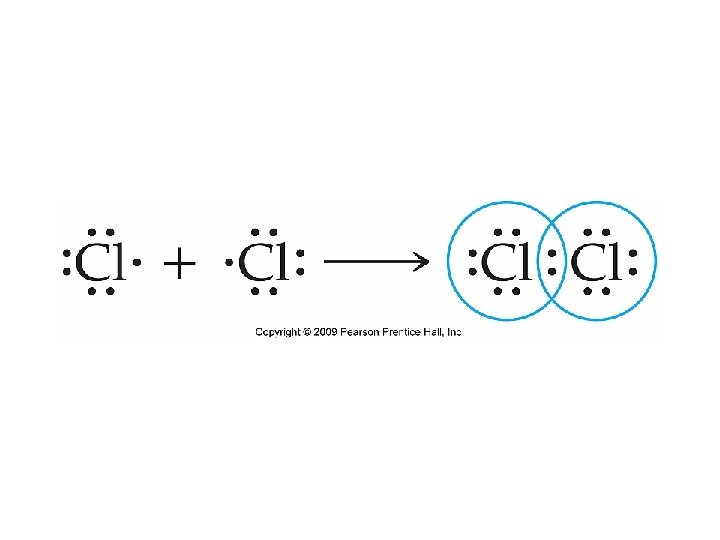
Which is the correct Lewis Structure for Chlorine? Either of these two!

Octet Rule • Atoms tend to gain, lose or share electrons until they are surrounded by 8 valence electrons. • In doing so, they achieve the configuration of a noble gas and achieve stability.

Ionic Bonding • Metals will lose electrons to achieve a stable outer valence level, and nonmetals will take those electrons to also achieve a stable outer valence. • Na. Cl is composed of Na+ and Cl- ions, arranged in a 3 -dimensional crystal.
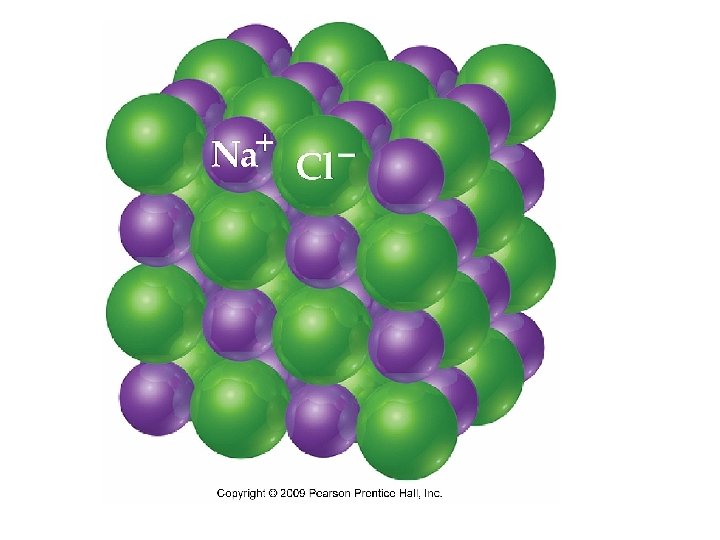
Formation of Na. Cl • There is an electron transfer between Na and Cl. • The metal, Na, has a low ioniziation energy and the nonmetal, Cl has a high electron affinity.
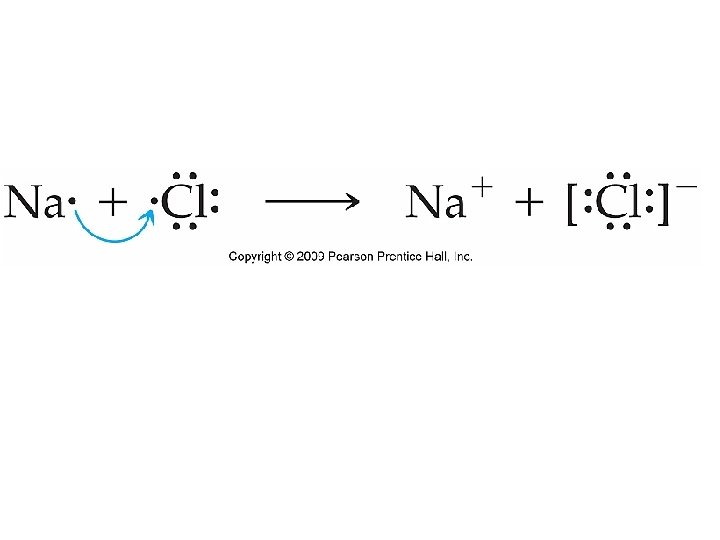
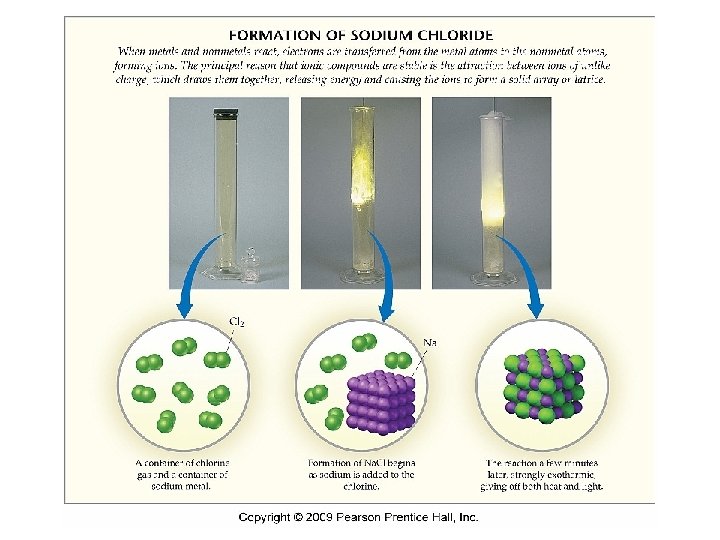

Energetics of Ionic Bonds • The formation of Na. Cl is extremely exothermic (ΔH°f = -410. 9 k. J). Why? • Loss of e- is always endothermic – takes energy to remove an electron from an orbital. When an e- is gained, the process is exothermic.

Lattice Energy • Lattice energy is a measure of just how much stabilization results from arranging oppositely charged ions in an ionic compound. Lattice energy is the energy required to completely separate a mole of solid ionic compound into its gaseous ions.
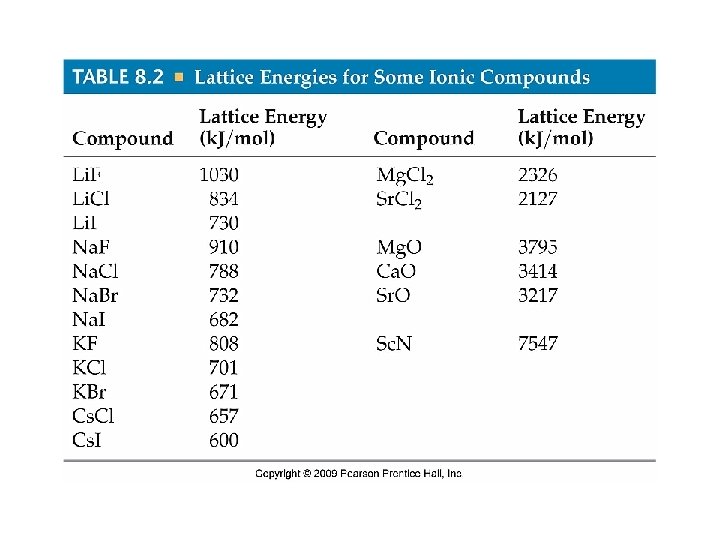

Lattice Energy • The magnitude of lattice energy depends on the charges of the ions, their sizes, and their arrangement in the solid. • For a given arrangement of ions, the lattice energy increases as the charges on the ions increases and as their radii decrease. • In forming ions, transition metals lose the valence shell e- first, then as many d e- as needed to reach the charge of the ion.

Because lattice energies are electrostatic in nature, two variables are involved in how big they are: (“wears the pants”) 1. the magnitude of the charges 2. the separation between the ions Charge can easily be twice as large (i. e. , 2+ and 2– vs. 1+ and 1–), but the separation never varies by that much. Put the following in order of increasing lattice energy: Li. Br, Fe. N, Cd. O. Li. Br < Cd. O < Fe. N Now these: Mg. S, Mg. Cl 2, Mg. O. Mg. Cl 2 < Mg. S < Mg. O
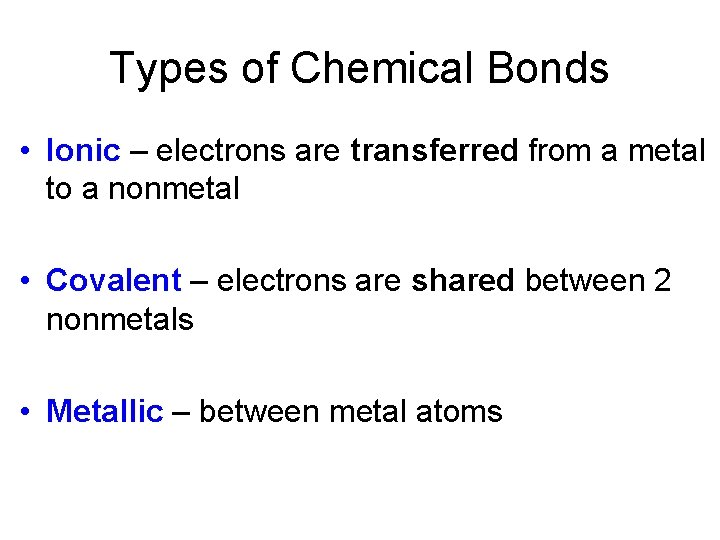
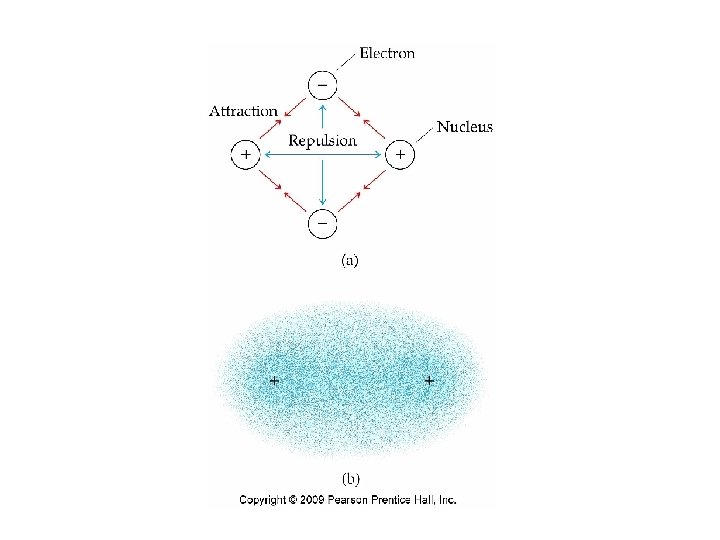
Covalent Bonding • A chemical bond formed by sharing a pair of electrons is called a covalent bond. • The attractive forces of the nuclei and electrons must overcome the repulsion between electrons and nuclei.
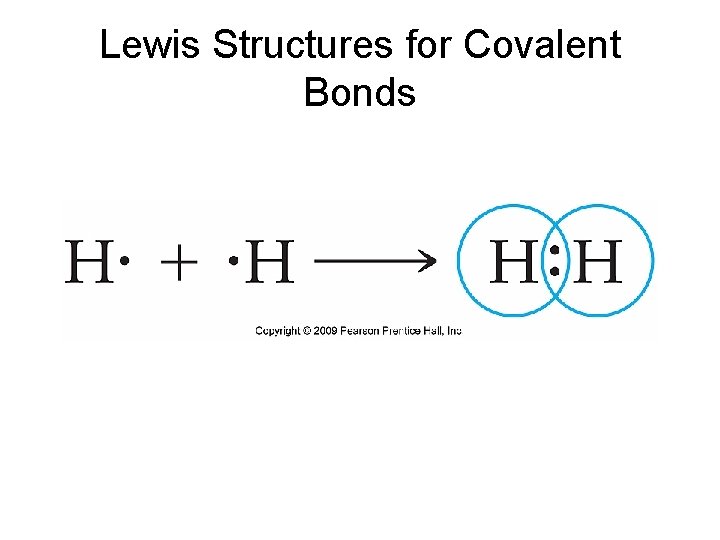
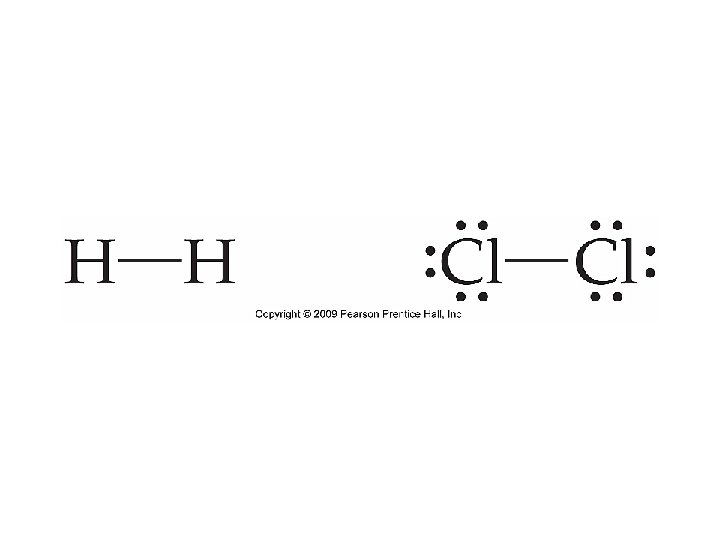
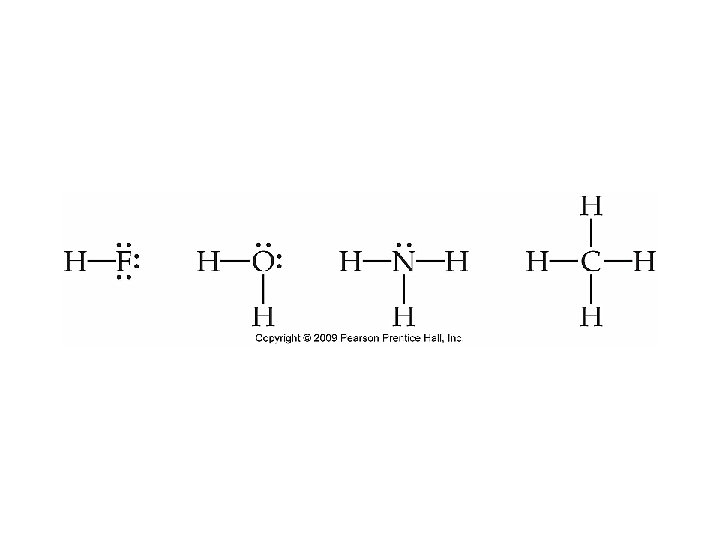
Lewis Structures for Covalent Bonds

Multiple Bonds
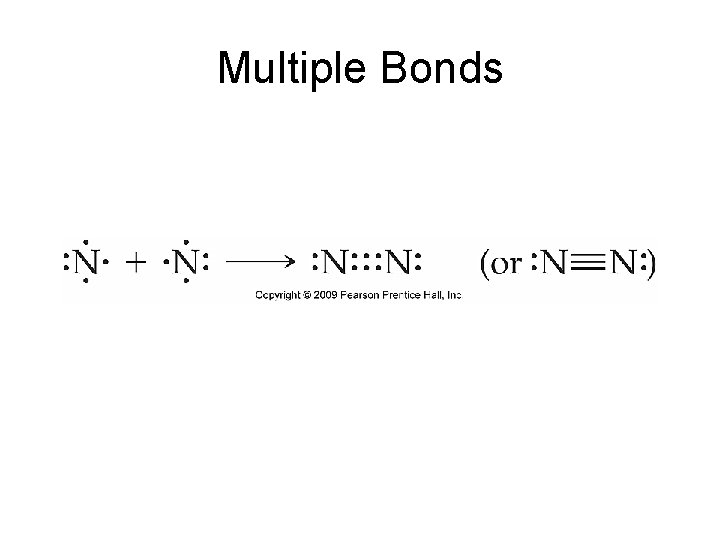
Multiple Bonds

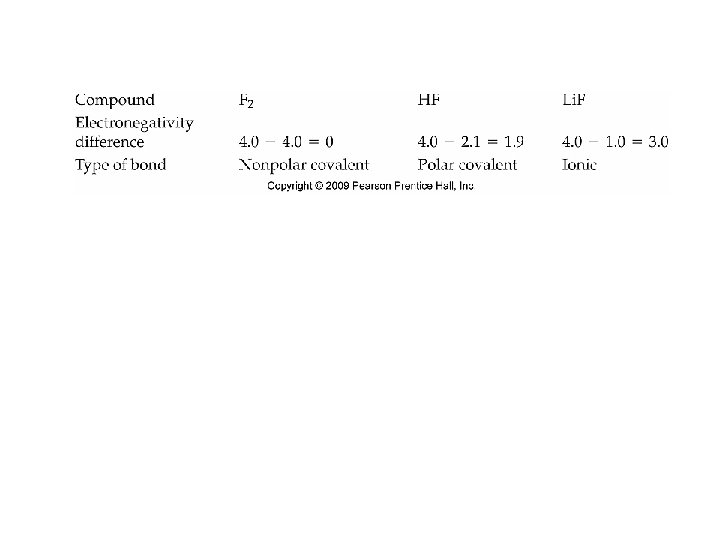
Bond Polarity and Electronegativity • Polar Covalent Bond – one atom pulls harder for the shared electrons than the other atom, forming a dipole. • The difference in electronegativity between the atoms must be less than 1. 7.
Bond Polarity • Non-polar Covalent Bonds – the atoms pull equally on the electrons. • The atoms must have the same electronegativity • The elements that have non-polar covalent bonds are the ones which are diatomic.
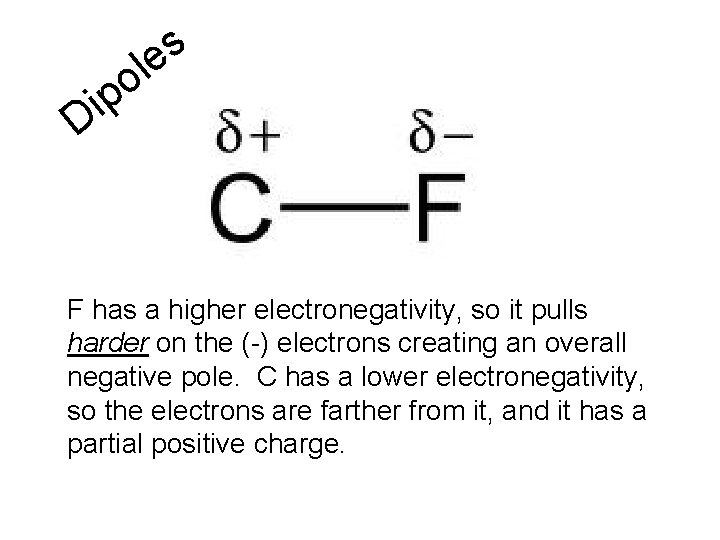
s le o p i D F has a higher electronegativity, so it pulls harder on the (-) electrons creating an overall negative pole. C has a lower electronegativity, so the electrons are farther from it, and it has a partial positive charge.
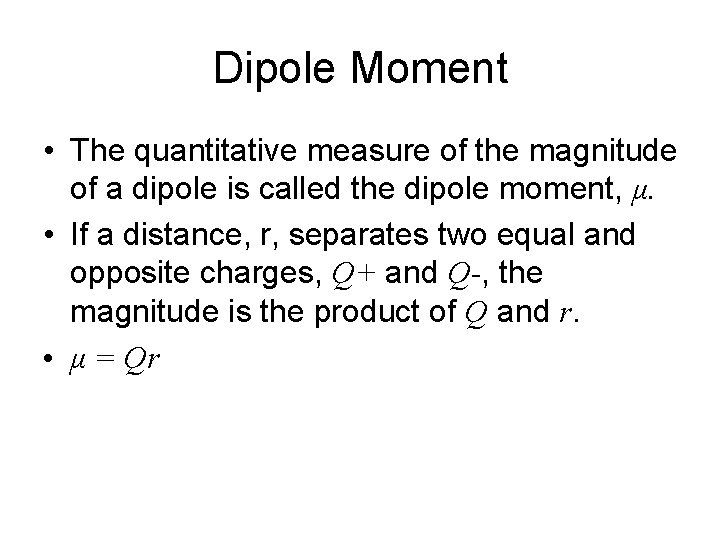
Dipole Moment • The quantitative measure of the magnitude of a dipole is called the dipole moment, μ. • If a distance, r, separates two equal and opposite charges, Q+ and Q-, the magnitude is the product of Q and r. • μ = Qr
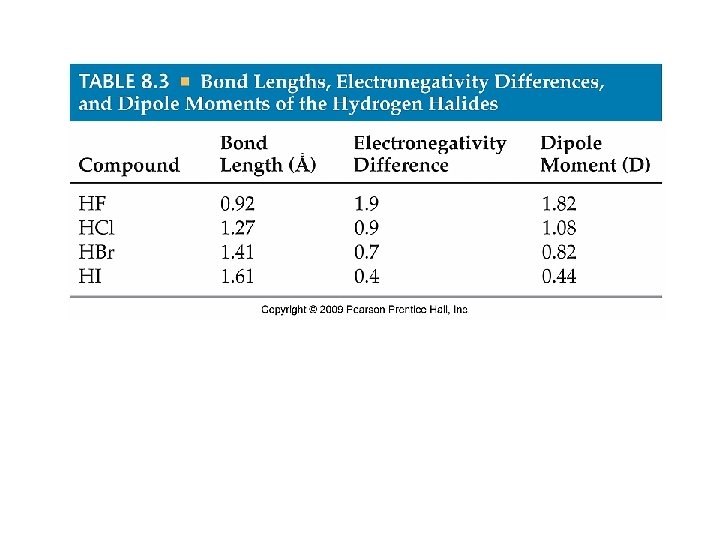
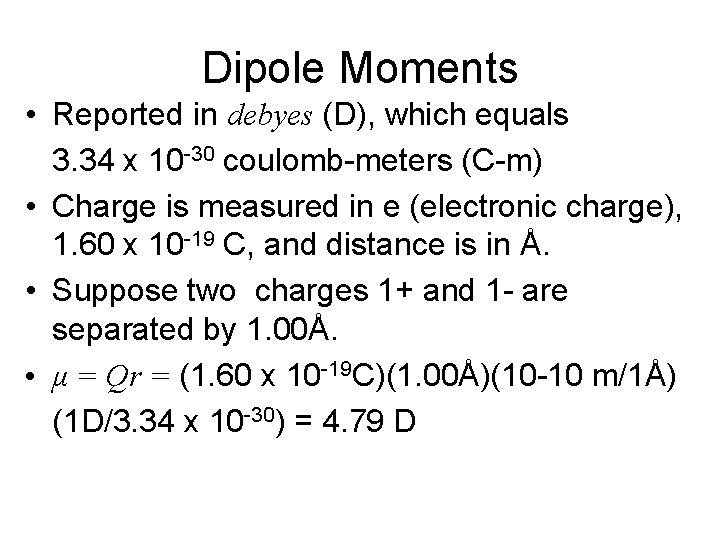
Dipole Moments • Reported in debyes (D), which equals 3. 34 x 10 -30 coulomb-meters (C-m) • Charge is measured in e (electronic charge), 1. 60 x 10 -19 C, and distance is in Å. • Suppose two charges 1+ and 1 - are separated by 1. 00Å. • μ = Qr = (1. 60 x 10 -19 C)(1. 00Å)(10 -10 m/1Å) (1 D/3. 34 x 10 -30) = 4. 79 D
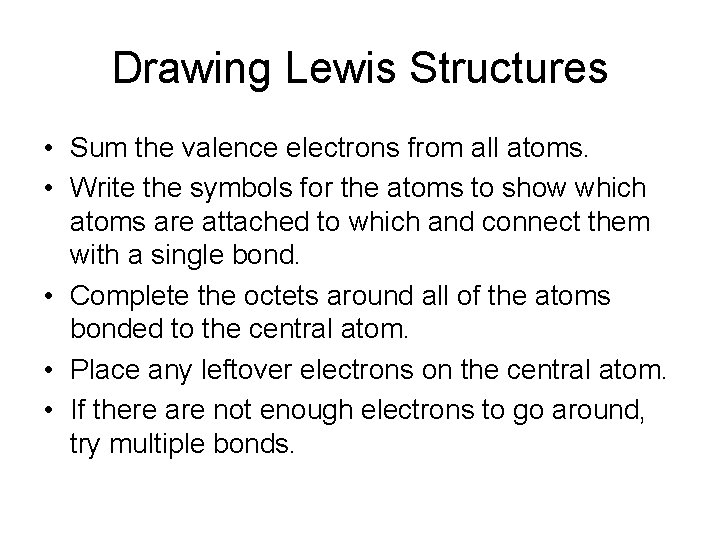
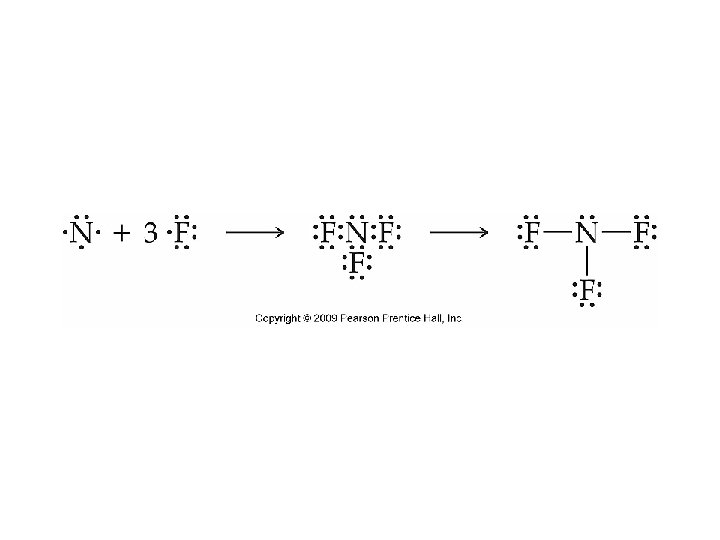
Drawing Lewis Structures • Sum the valence electrons from all atoms. • Write the symbols for the atoms to show which atoms are attached to which and connect them with a single bond. • Complete the octets around all of the atoms bonded to the central atom. • Place any leftover electrons on the central atom. • If there are not enough electrons to go around, try multiple bonds.
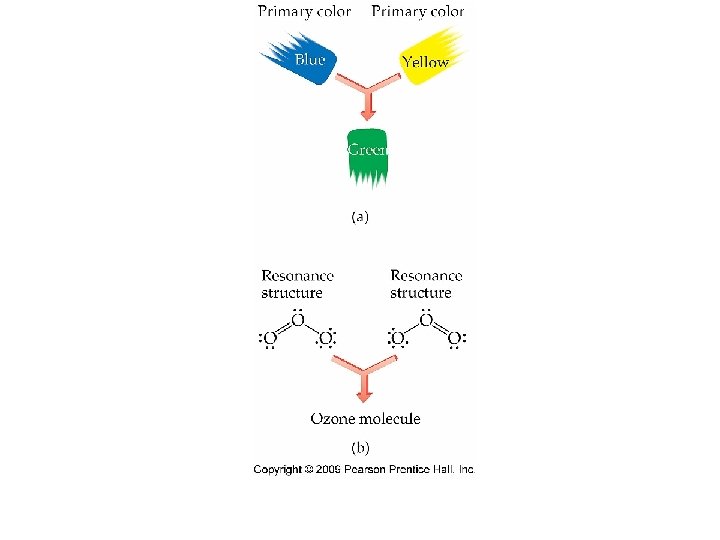
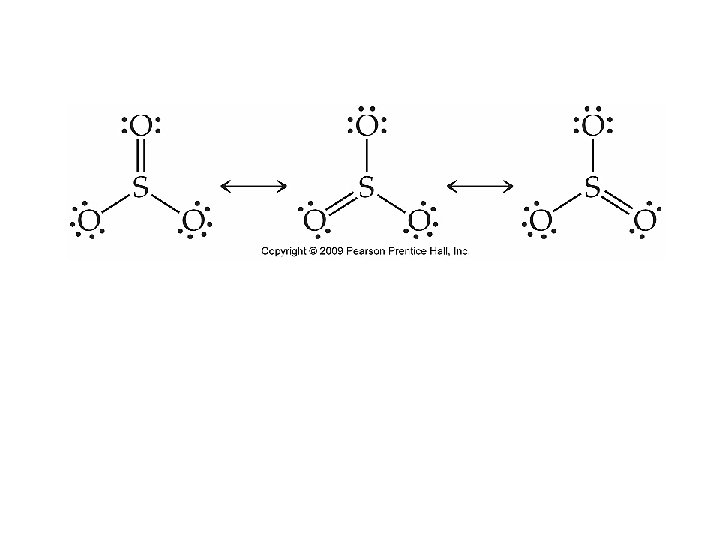
Resonance Structures • Multiple, but equally good representations for individual Lewis structures can be drawn for a molecule. • The resonance structures are “averaged” to give a more accurate description of the molecule.
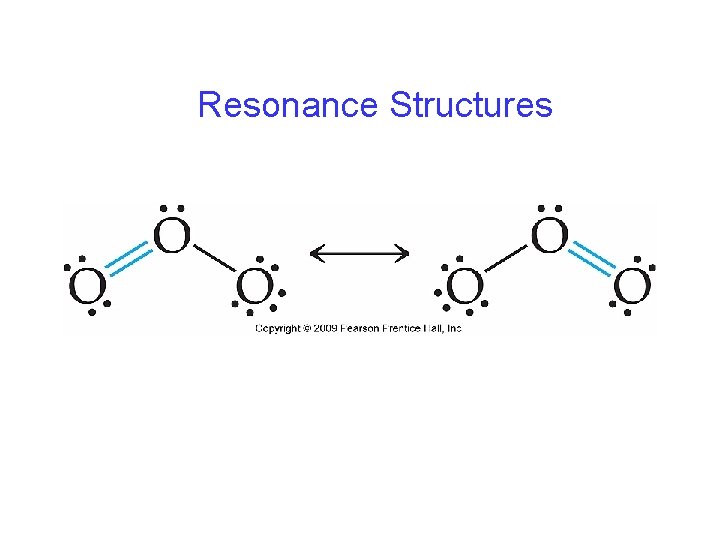
Resonance Structures
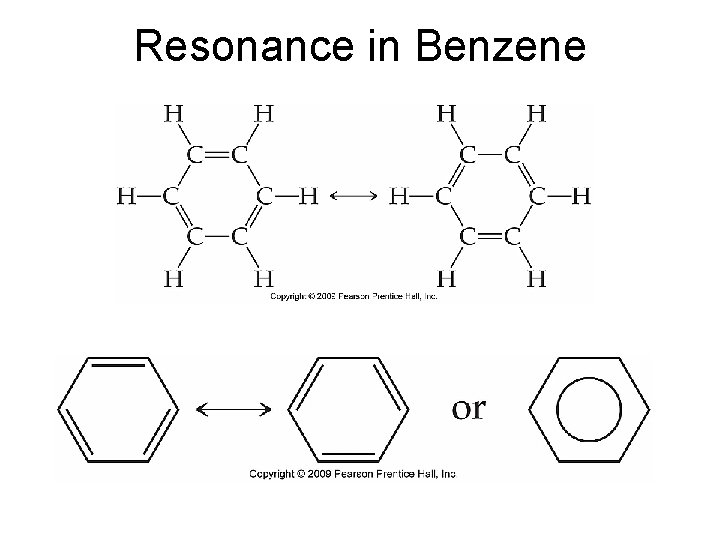
Resonance in Benzene
Exceptions to the Octet Rule • The octet rule has limits when dealing with some of the transition metals and some covalent compounds. – Molecules and polyatomic ions with an odd number of electrons – Molecules and polyatomic ions in which an atom has fewer than an octet of valence electrons – Molecules and polyatomic ions in which an atom has more than an octet of valence electrons
Strengths of Covalent Bonds • The stability of a molecule is related to the strengths of the bonds it contains. • Bond enthalpy is the enthalpy change, ΔH, for breaking a particular bond in one mole of a gaseous substance. • ΔHrxn = Σ(bond enthalpies of bonds broken) – Σ(bond enthalpies of bonds formed)
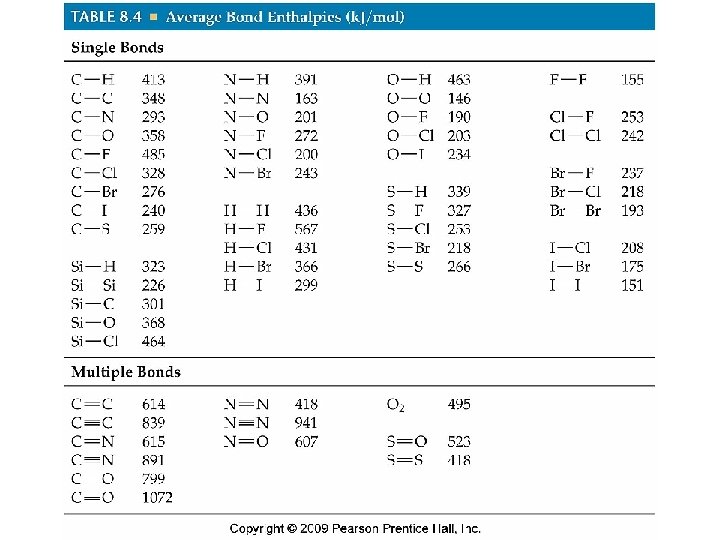
Bond Enthalpies, continued • H—CH 3 + Cl—Cl → Cl—CH 3 + H—Cl • ΔHrxn = [D(C—H) + D(Cl—Cl)] – [D(C—Cl) + D(H—Cl)] • = (413 k. J + 242 k. J) – (328 k. J + 431 k. J) = -104 k. J • This reaction is exothermic because the bonds in the products are stronger than the bonds in the reactants, and the ΔHrxn value is negative. • These are often averaged values and provide an estimate.
- Slides: 45