Chemical Bonding Chapter 6 Imran Syakir Mohamad Chemistry
Chemical Bonding Chapter 6 Imran Syakir Mohamad Chemistry DMCU 1233 1
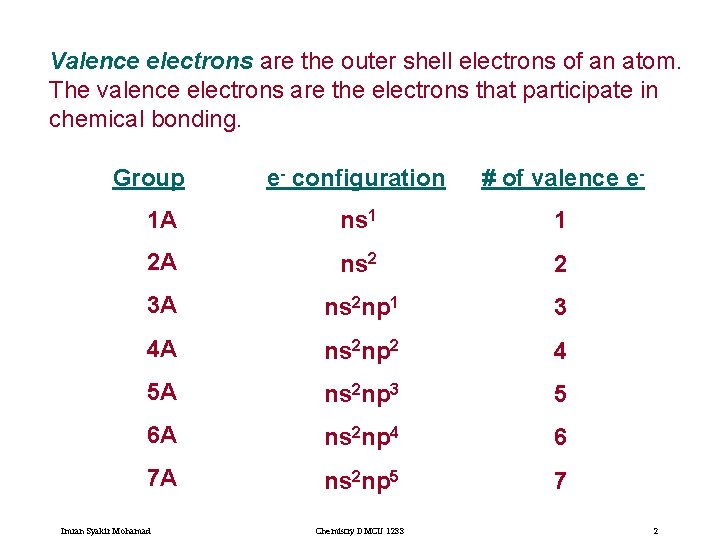
Valence electrons are the outer shell electrons of an atom. The valence electrons are the electrons that participate in chemical bonding. Group e- configuration # of valence e- 1 A ns 1 1 2 A ns 2 2 3 A ns 2 np 1 3 4 A ns 2 np 2 4 5 A ns 2 np 3 5 6 A ns 2 np 4 6 7 A ns 2 np 5 7 Imran Syakir Mohamad Chemistry DMCU 1233 2
Imran Syakir Mohamad Chemistry DMCU 1233 3
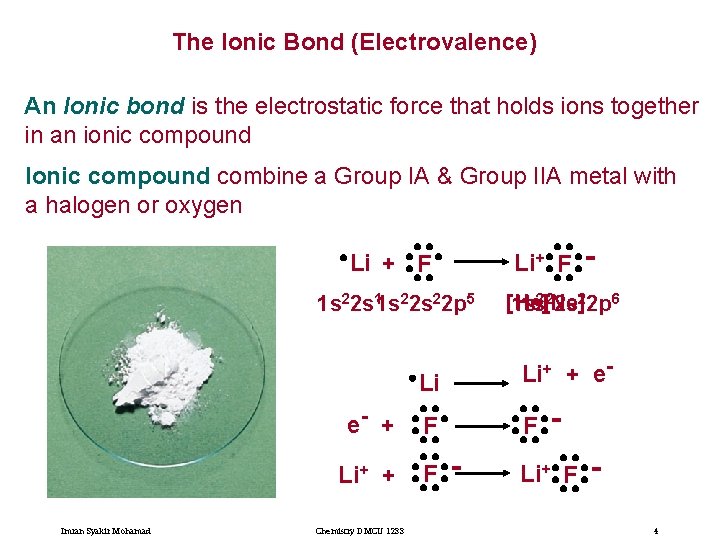
The Ionic Bond (Electrovalence) An Ionic bond is the electrostatic force that holds ions together in an ionic compound Ionic compound combine a Group IA & Group IIA metal with a halogen or oxygen Li + F 1 s 22 s 11 s 22 p 5 e- + Li+ + Imran Syakir Mohamad Chemistry DMCU 1233 Li+ F [He] 1 s 1 s 2[2 Ne] 2 s 22 p 6 Li Li+ + e- F F - Li+ F 4
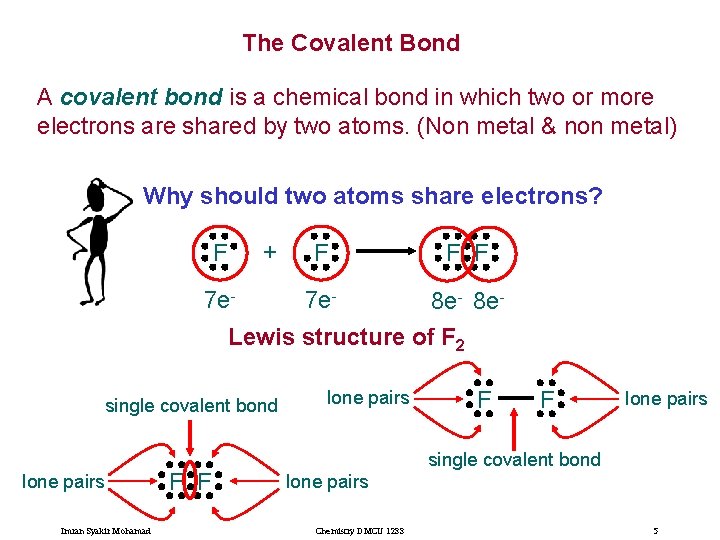
The Covalent Bond A covalent bond is a chemical bond in which two or more electrons are shared by two atoms. (Non metal & non metal) Why should two atoms share electrons? + F 7 e- F F F 7 e- 8 e. Lewis structure of F 2 single covalent bond lone pairs Imran Syakir Mohamad F F lone pairs single covalent bond lone pairs Chemistry DMCU 1233 5
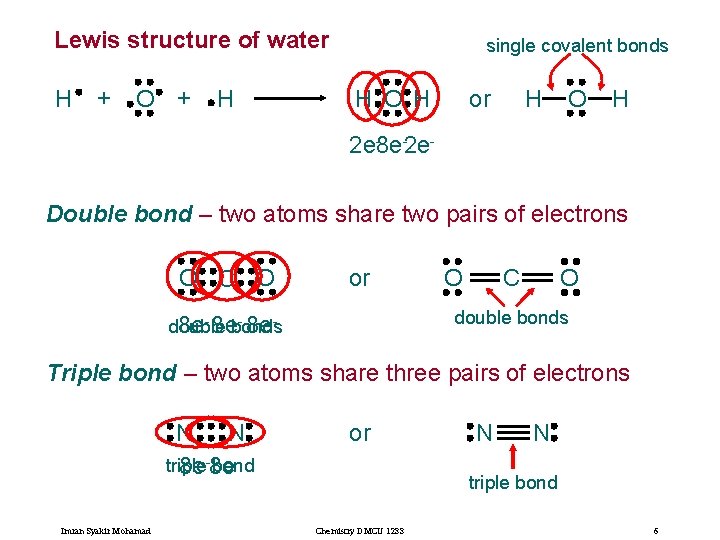
Lewis structure of water H + O + H single covalent bonds H O H or H O H 2 e-8 e-2 e. Double bond – two atoms share two pairs of electrons O C O or O O C double bonds - 8 edouble 8 e- 8 ebonds Triple bond – two atoms share three pairs of electrons N N triple bond 8 e-8 e Imran Syakir Mohamad or N N triple bond Chemistry DMCU 1233 6
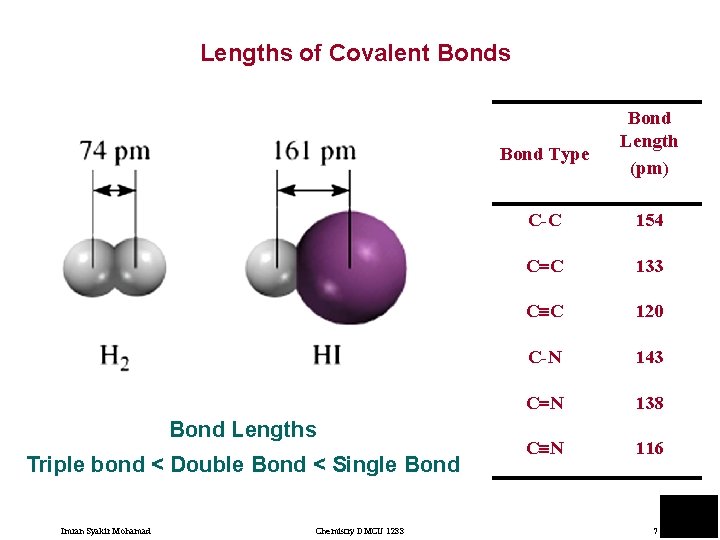
Lengths of Covalent Bonds Bond Type Bond Lengths Triple bond < Double Bond < Single Bond Imran Syakir Mohamad Chemistry DMCU 1233 Bond Length (pm) C-C 154 C C 133 C C 120 C-N 143 C N 138 C N 116 7
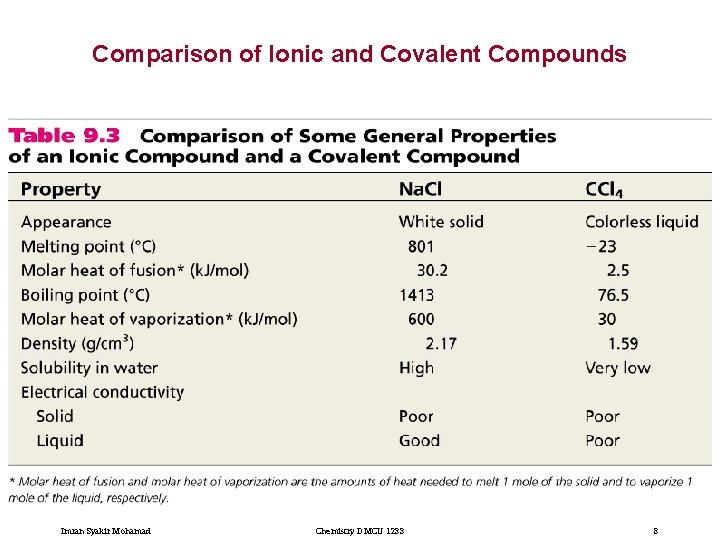
Comparison of Ionic and Covalent Compounds Imran Syakir Mohamad Chemistry DMCU 1233 8
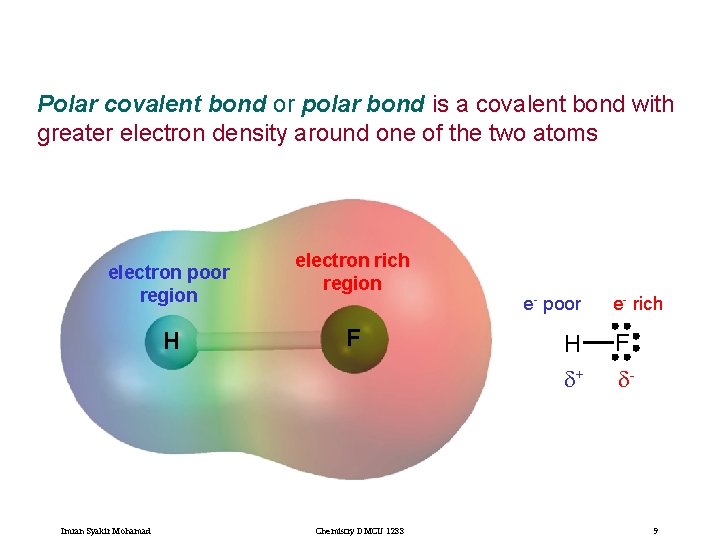
Polar covalent bond or polar bond is a covalent bond with greater electron density around one of the two atoms electron poor region H Imran Syakir Mohamad electron rich region F Chemistry DMCU 1233 e- poor H d+ e- rich F d- 9
Electronegativity is the ability of an atom to attract toward itself the electrons in a chemical bond. Electron Affinity - measurable, Cl is highest Electronegativity - relative, F is highest Both are related but different concepts. EA refers to an isolated atom and E refers to an atom in chemical bond. Usually, EA > then E >. Imran Syakir Mohamad Chemistry DMCU 1233 10
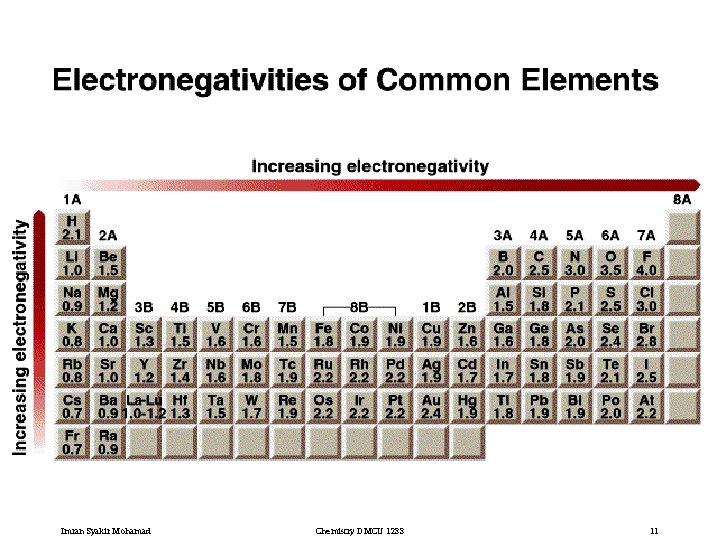
Imran Syakir Mohamad Chemistry DMCU 1233 11
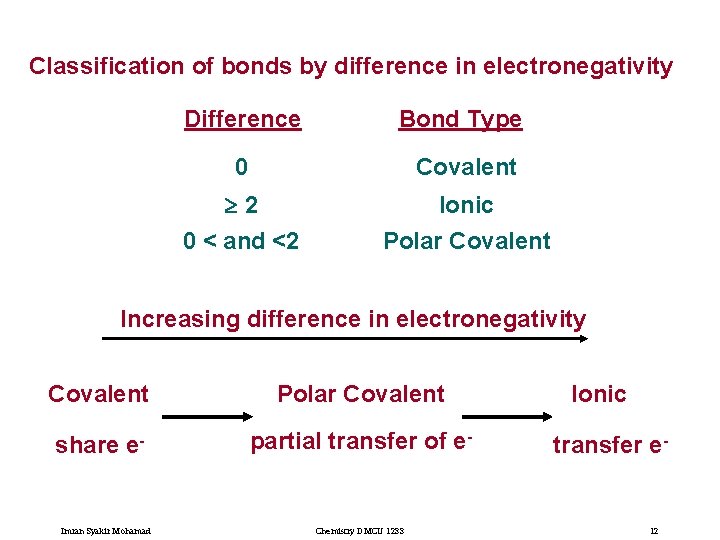
Classification of bonds by difference in electronegativity Difference Bond Type 0 Covalent 2 Ionic Polar Covalent 0 < and <2 Increasing difference in electronegativity Covalent Polar Covalent share e- partial transfer of e- Imran Syakir Mohamad Chemistry DMCU 1233 Ionic transfer e- 12

Classify the following bonds as ionic, polar covalent, or covalent: The bond in Cs. Cl; the bond in H 2 S; and the NN bond in H 2 NNH 2. Imran Syakir Mohamad Chemistry DMCU 1233 13
Intermolecular Forces Intermolecular forces are attractive forces between molecules. Intramolecular forces hold atoms together in a molecule. Intermolecular vs Intramolecular • 41 k. J to vaporize 1 mole of water (inter) • 930 k. J to break all O-H bonds in 1 mole of water (intra) Generally, intermolecular forces are much weaker than intramolecular forces. Imran Syakir Mohamad “Measure” of intermolecular force Chemistry DMCU 1233 boiling point melting point DHvap 14
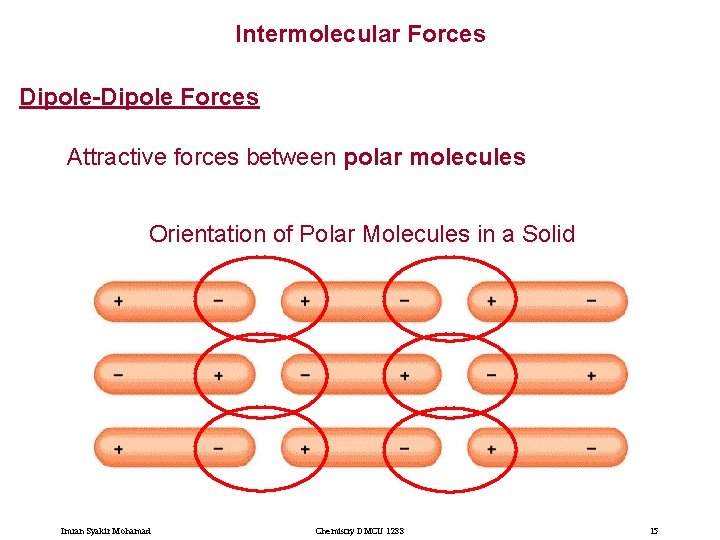
Intermolecular Forces Dipole-Dipole Forces Attractive forces between polar molecules Orientation of Polar Molecules in a Solid Imran Syakir Mohamad Chemistry DMCU 1233 15
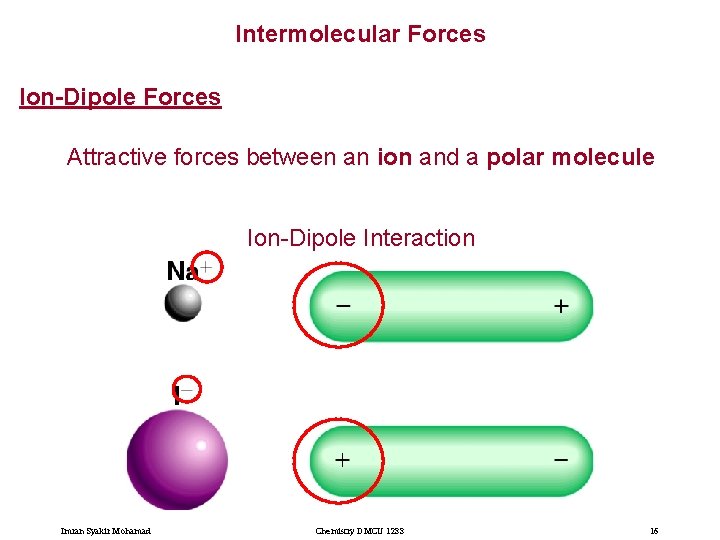
Intermolecular Forces Ion-Dipole Forces Attractive forces between an ion and a polar molecule Ion-Dipole Interaction Imran Syakir Mohamad Chemistry DMCU 1233 16
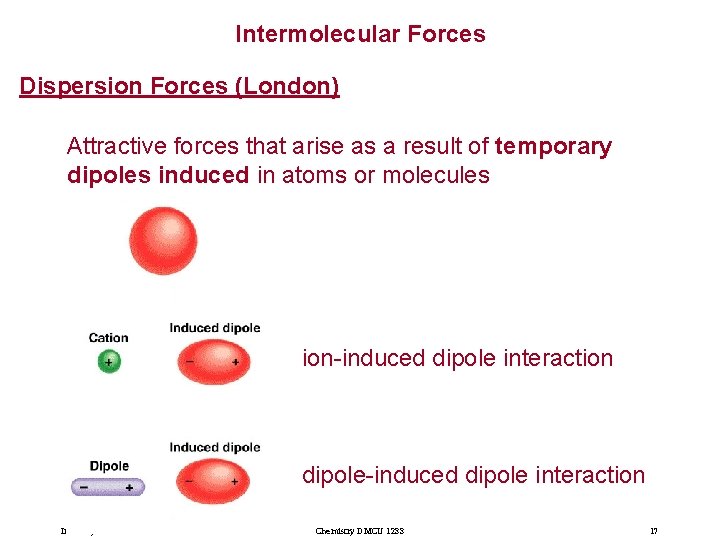
Intermolecular Forces Dispersion Forces (London) Attractive forces that arise as a result of temporary dipoles induced in atoms or molecules ion-induced dipole interaction dipole-induced dipole interaction Imran Syakir Mohamad Chemistry DMCU 1233 17
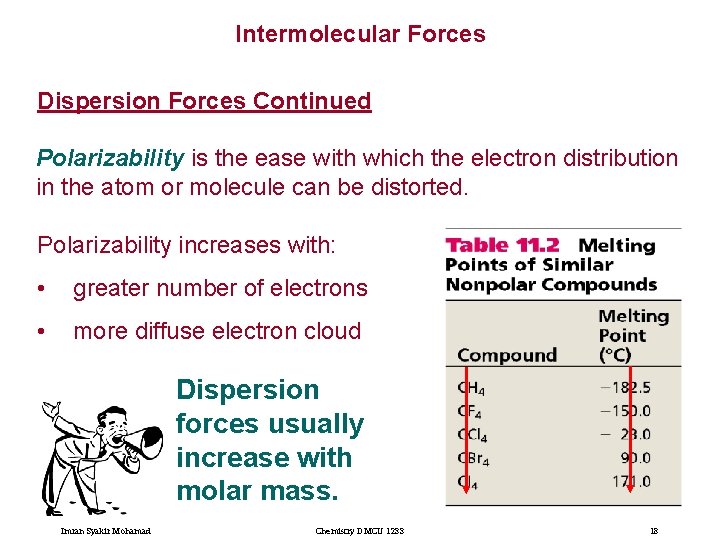
Intermolecular Forces Dispersion Forces Continued Polarizability is the ease with which the electron distribution in the atom or molecule can be distorted. Polarizability increases with: • greater number of electrons • more diffuse electron cloud Dispersion forces usually increase with molar mass. Imran Syakir Mohamad Chemistry DMCU 1233 18

What type(s) of intermolecular forces exist between each of the following molecules? HBr CH 4 SO 2 Imran Syakir Mohamad Chemistry DMCU 1233 19
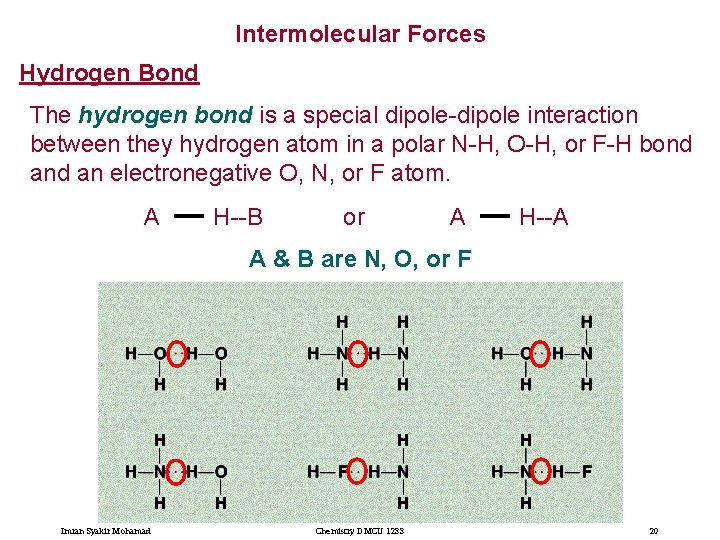
Intermolecular Forces Hydrogen Bond The hydrogen bond is a special dipole-dipole interaction between they hydrogen atom in a polar N-H, O-H, or F-H bond an electronegative O, N, or F atom. A H--B or A H--A A & B are N, O, or F Imran Syakir Mohamad Chemistry DMCU 1233 20
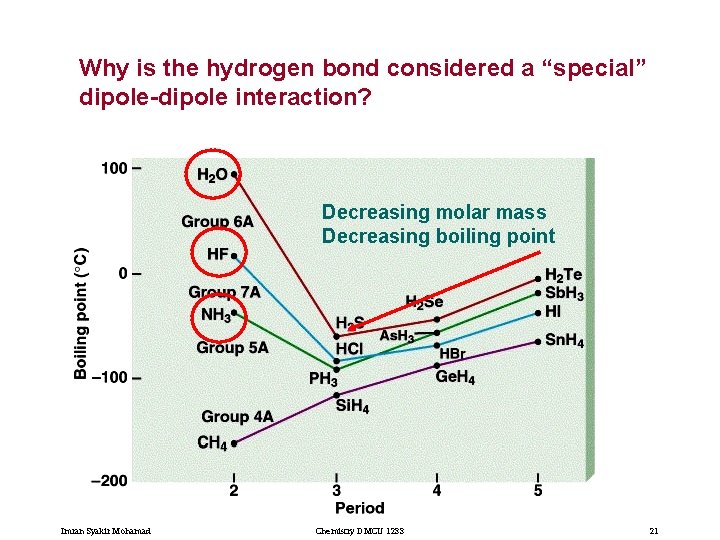
Why is the hydrogen bond considered a “special” dipole-dipole interaction? Decreasing molar mass Decreasing boiling point Imran Syakir Mohamad Chemistry DMCU 1233 21
- Slides: 21