Chemical Bonding and Atomic Structure Mendeleev Who is

Chemical Bonding and Atomic Structure Mendeleev Who is Mendeleev?

Lesson objectives • Explain how Mendeleev: a) arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b) used his table to predict the existence and properties of some elements not then discovered • Classify elements as metals and non-metals according to their position in the periodic table
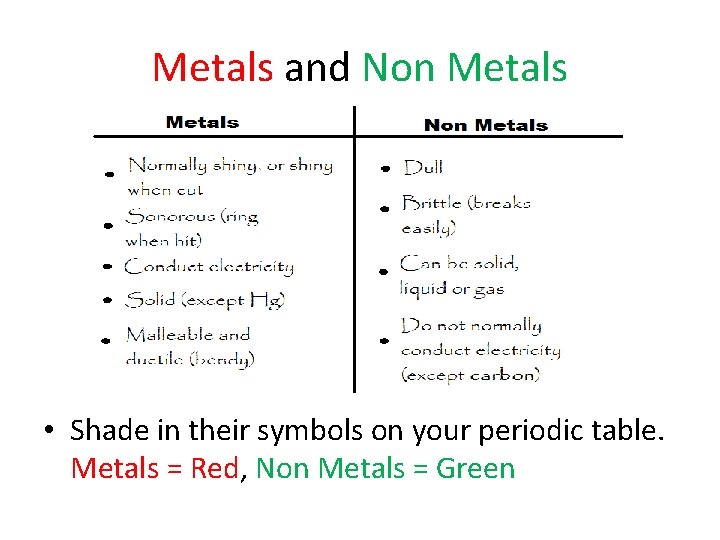
Metals and Non Metals • Shade in their symbols on your periodic table. Metals = Red, Non Metals = Green
Structure of the atom What is an element?
Lesson objectives • 1. 3 Describe the structure of an atom • 1. 5 Describe atoms of a given element as having the same number of protons in the nucleus, and that this number is unique to that element • 1. 6 Recall the relative charge and relative mass of: a) a proton b) a neutron c) an electron
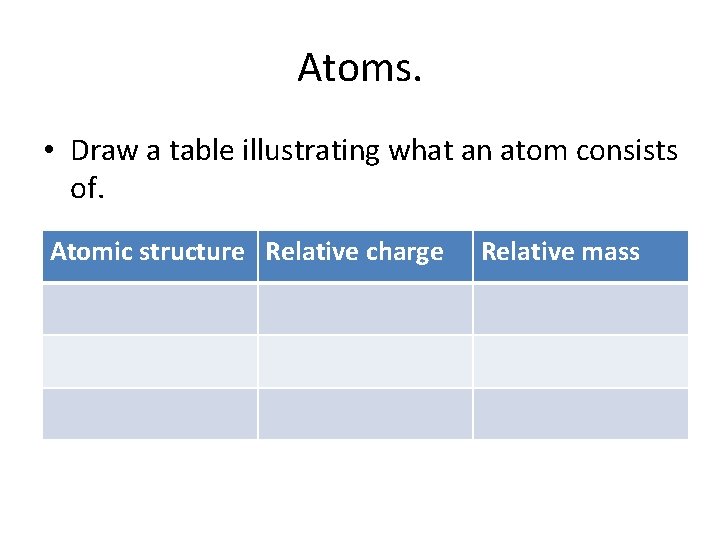
Atoms. • Draw a table illustrating what an atom consists of. Atomic structure Relative charge Relative mass
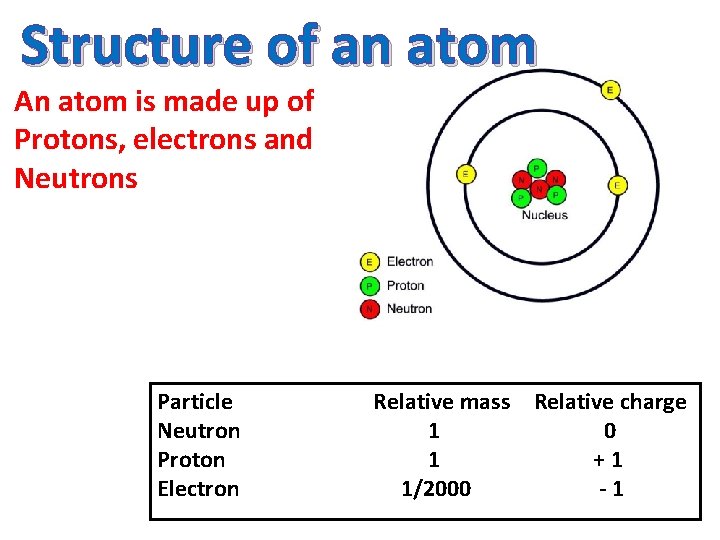
Structure of an atom An atom is made up of Protons, electrons and Neutrons Particle Neutron Proton Electron Relative mass Relative charge 1 0 1 +1 1/2000 -1
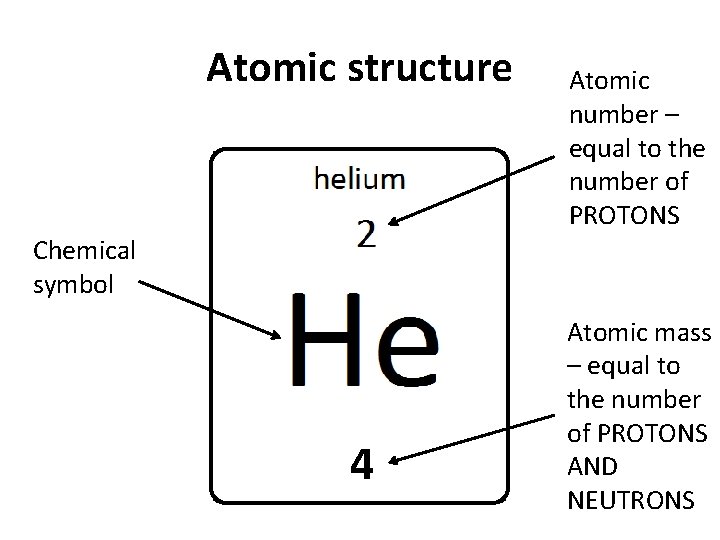
Atomic structure Atomic number – equal to the number of PROTONS Chemical symbol 4 Atomic mass – equal to the number of PROTONS AND NEUTRONS
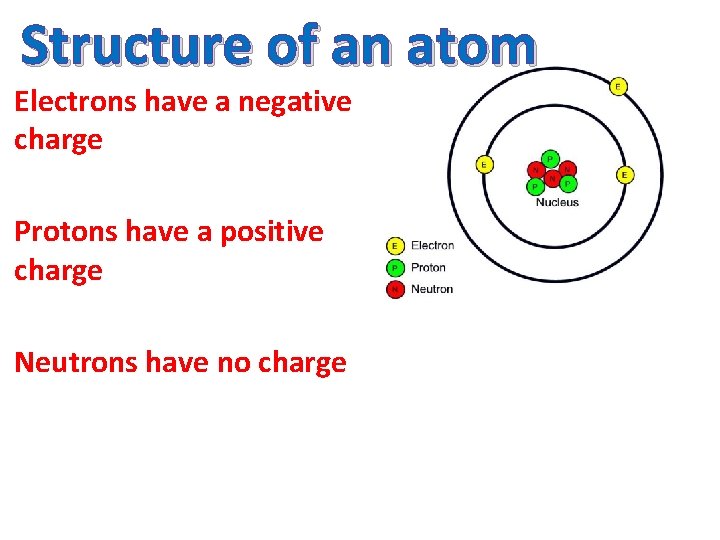
Structure of an atom Electrons have a negative charge Protons have a positive charge Neutrons have no charge
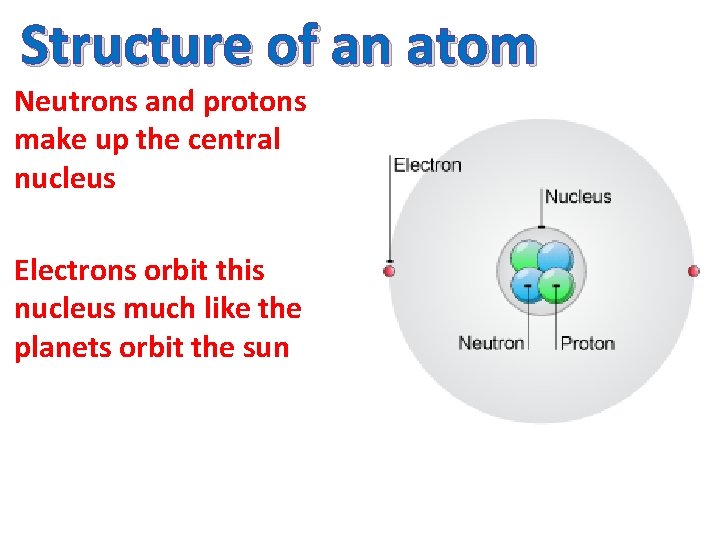
Structure of an atom Neutrons and protons make up the central nucleus Electrons orbit this nucleus much like the planets orbit the sun
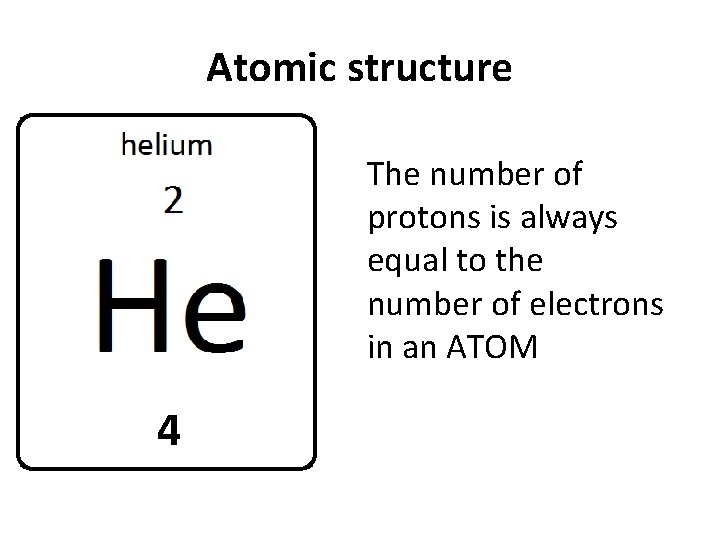
Atomic structure The number of protons is always equal to the number of electrons in an ATOM 4
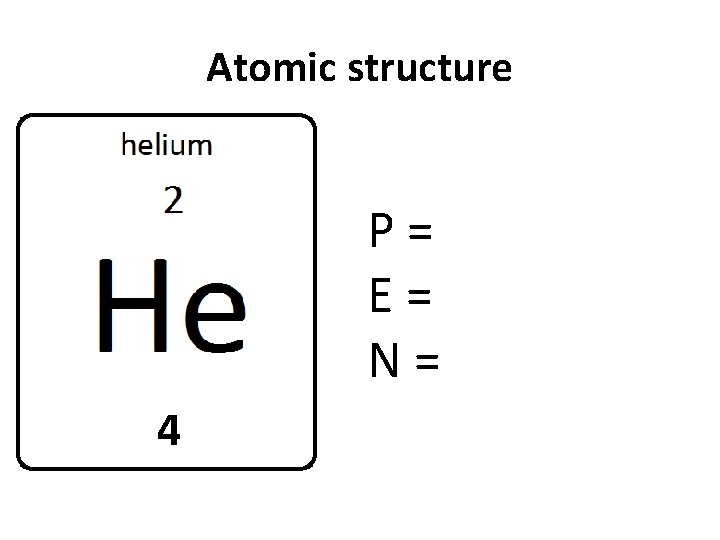
Atomic structure P= E= N= 4
Atomic structure • Using the periodic table record the atomic structure for the first 20 elements set out each one as below: – Element – Number of protons – Number of electrons – Number of neutrons
Why are isotopes different to atoms? • Every atom of a particular element has the same number of protons as any other atom of the same element. E. g. a carbon atom always has 6 protons. • The number of protons in an atom is called the ‘atomic number’ • However, atoms of the same element can have different numbers of neutrons. An isotope is atoms of the same element (so they have the same number of protons) with different numbers of neutrons. • The total number of protons and neutrons in an atom is called the ‘mass number’ Carbon is a good example of an atom with isotopes: Carbon 12, Carbon 13 and Carbon 14
- Slides: 14