Chemical Behavior of Metals Chapter 3 Activity 3

Chemical Behavior of Metals Chapter 3: Activity 3

Solutions • Solid copper chloride is added to water • Cu. Cl 2(s) → Cu+2(aq) + 2 Cl-1(aq)

Which Metals Won’t Corrode? What would happen if solid metal ZINC was placed in a copper chloride solution? Zn (s) + Cu(Cl)2 (aq) ?

Single Replacement Reaction When solid ZINC was placed in a copper chloride aqueous solution…. . Zn (s) + Cu(Cl)2 (aq) Zn. Cl 2 (aq)+ Cu (s)
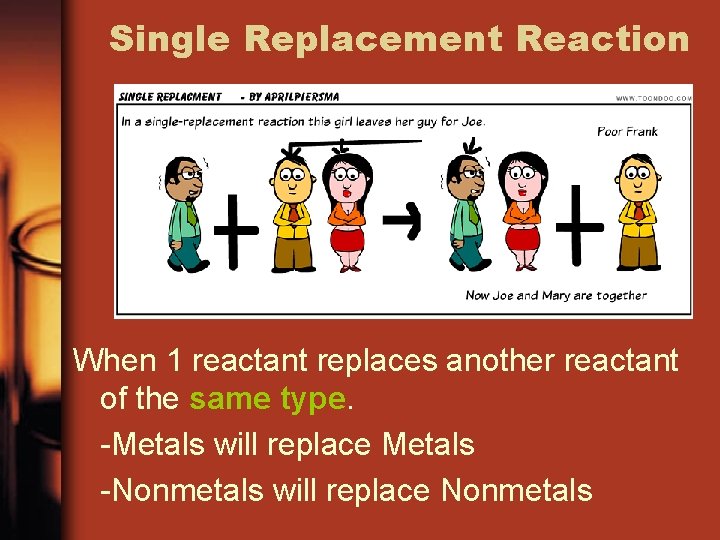
Single Replacement Reaction When 1 reactant replaces another reactant of the same type. -Metals will replace Metals -Nonmetals will replace Nonmetals

Online animation
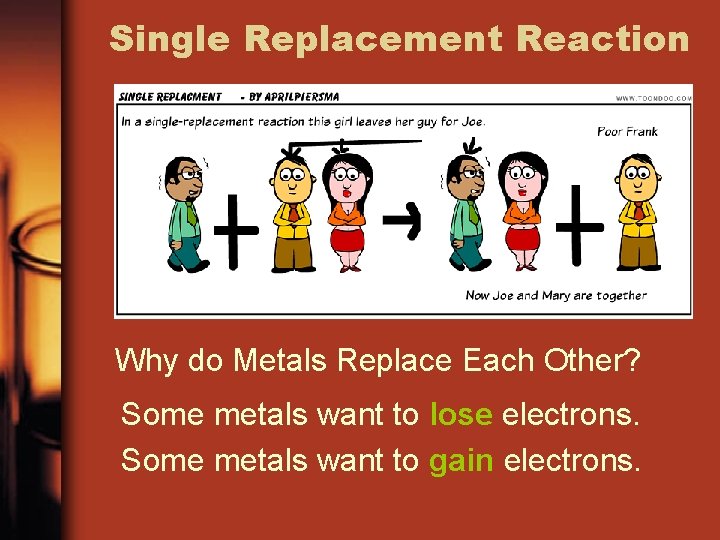
Single Replacement Reaction Why do Metals Replace Each Other? Some metals want to lose electrons. Some metals want to gain electrons.
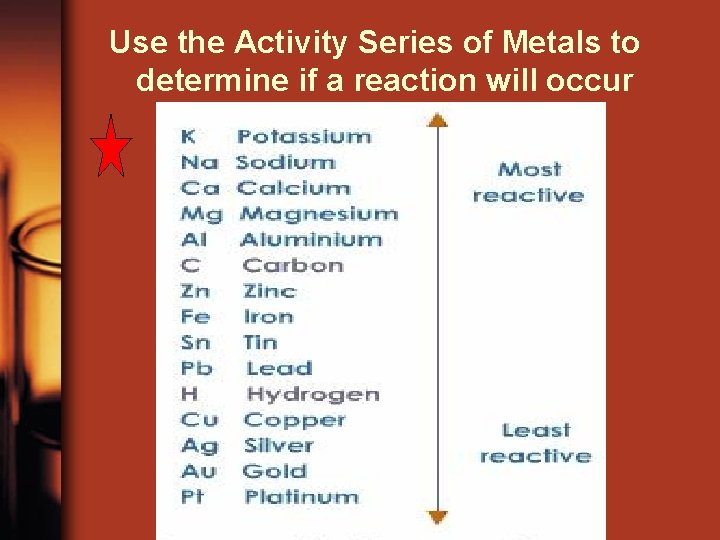
Use the Activity Series of Metals to determine if a reaction will occur

Use the Activity Series of Metals to determine if a reaction will occur Is Zn higher than Cu on the list? Li K Ba Sr Ca Na Mg Al Mn Zn Fe Cd Co Ni Sn Pb H Cu Ag Hg Au
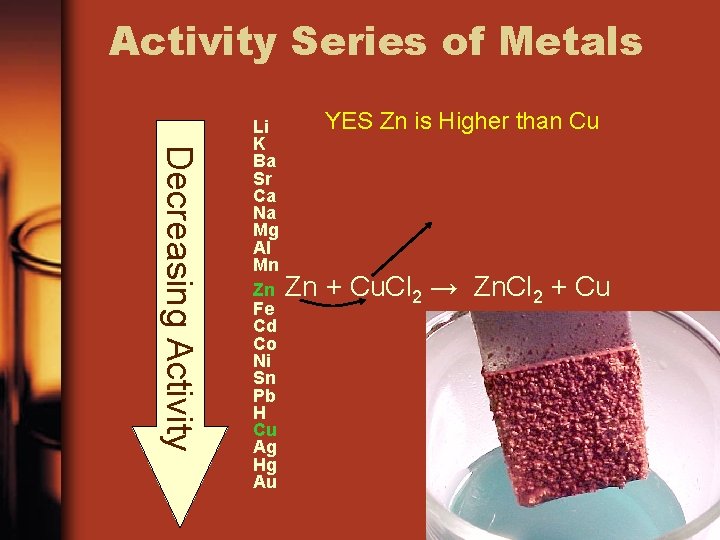
Activity Series of Metals Decreasing Activity Li K Ba Sr Ca Na Mg Al Mn Zn Fe Cd Co Ni Sn Pb H Cu Ag Hg Au YES Zn is Higher than Cu Zn + Cu. Cl 2 → Zn. Cl 2 + Cu
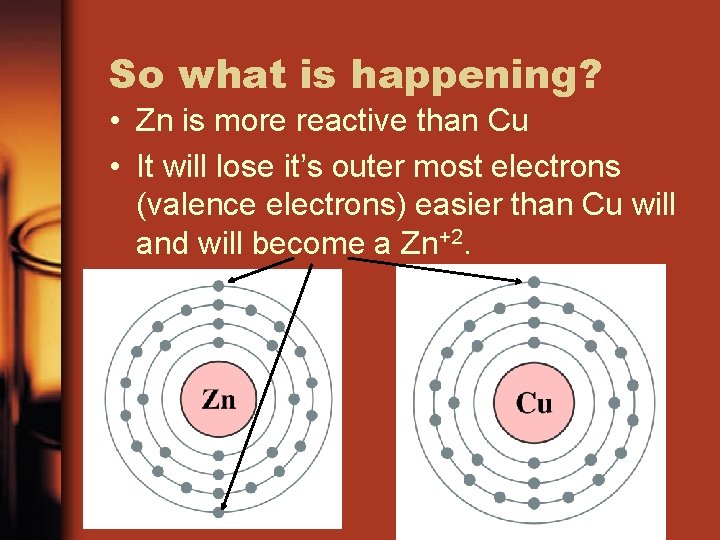
So what is happening? • Zn is more reactive than Cu • It will lose it’s outer most electrons (valence electrons) easier than Cu will and will become a Zn+2.

Cl-1 Cl Cu+2
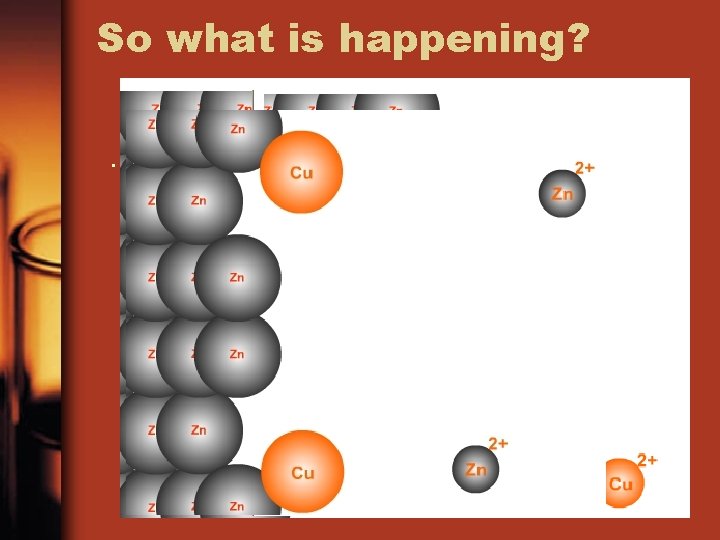
So what is happening? .
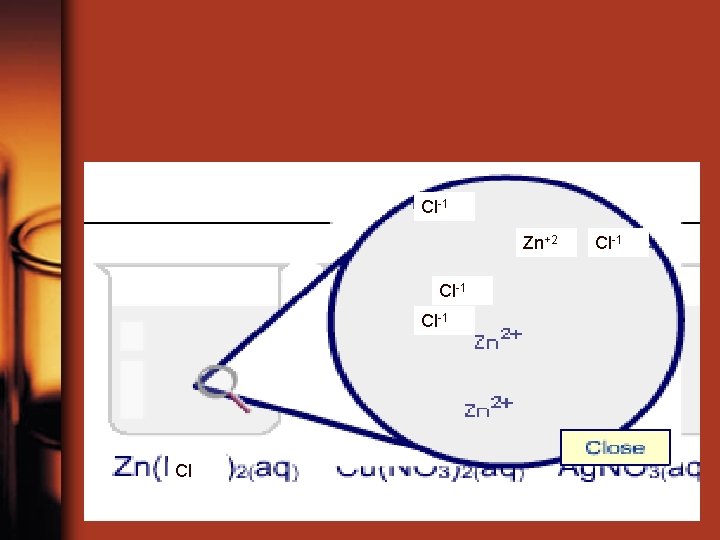
Cl-1 Zn+2 Cl-1 Cl Cl-1

Recap 1. In solution, metals are floating around by themselves as ions 2. Metals that are higher on the Activity Series are more reactive than metals lower on the list 3. Metals that are higher are more reactive and want to lose their valence electrons

Yesterday’s Activity • Solid aluminum was added to a solution of copper chloride • Al(s) + Cu. Cl 2(aq) → Cu(s) + Al. Cl 3(aq) • So, solid aluminum went into solution and copper came out of solution!!!! • WHY? ? ? ?

Some practice • Will this reaction happen? Cu + Ag. NO 3→ ? Yes, Cu is higher on the list Cu + Ag. NO 3→ Ag + Cu(NO 3)2 So, after the reaction, silver is now the solid!

Some practice • Will this reaction happen? Cu + Pb(NO 3)2→ ? No, Cu is lower on the list Cu + Pb(NO 3)2 → No Reaction So, copper is still a solid
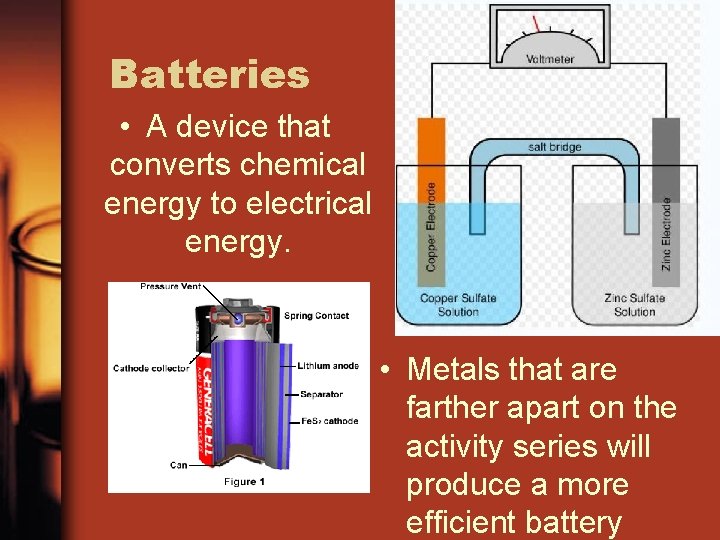
Batteries • A device that converts chemical energy to electrical energy. • Metals that are farther apart on the activity series will produce a more efficient battery
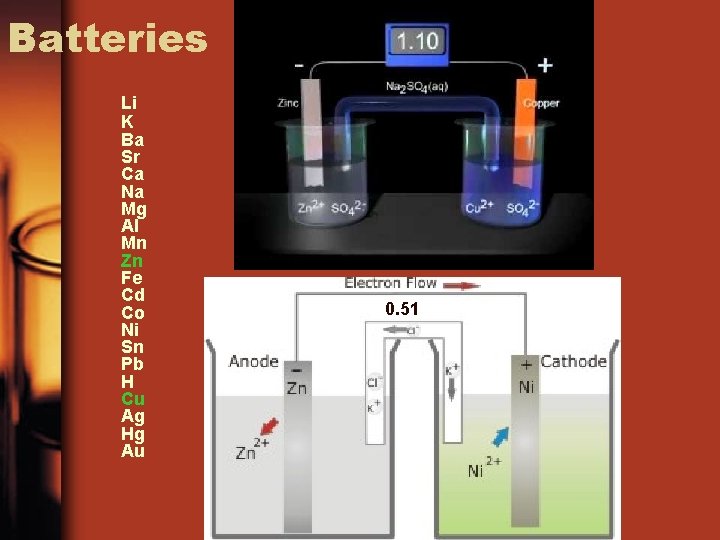
Batteries Li K Ba Sr Ca Na Mg Al Mn Zn Fe Cd Co Ni Sn Pb H Cu Ag Hg Au 0. 51

How Batteries Work Video

Car Battery



Why do metals react? • Metals that are more reactive want to LOSE electrons – This is called OXIDATION 0 +1 0 +2 Cu + Ag. NO 3→ Ag + Cu(NO 3)2 • When the less reactive metal GAIN these lost electrons – This is called REDUCTION • SO… Cu Lost Electrons = Oxidation Ag Gained Electrons = Reduction

REDOX REACTIONS • LEO the lion goes GER • Losing Electrons is Oxidation • Gaining Electrons is Reduction

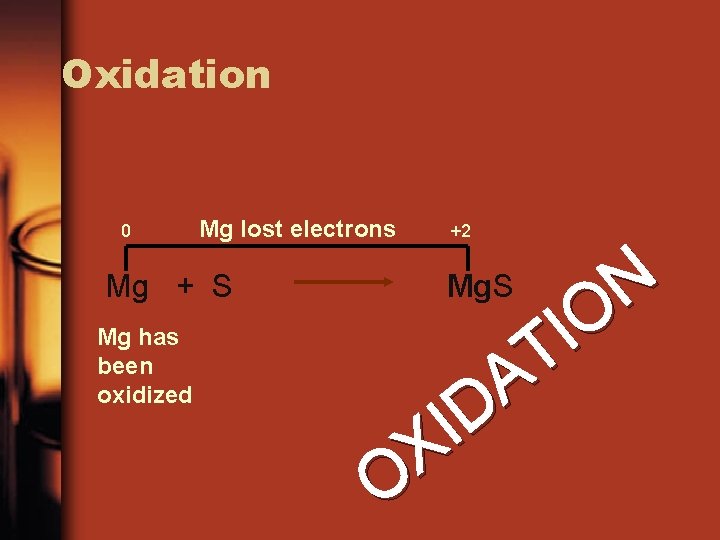
Oxidation 0 Mg lost electrons Mg + S Mg has been oxidized +2 N O I T A D I X O Mg. S

Reduction 0 S gained electrons Mg + S Sulfur has been reduced -2 N O I T C U D E R Mg. S
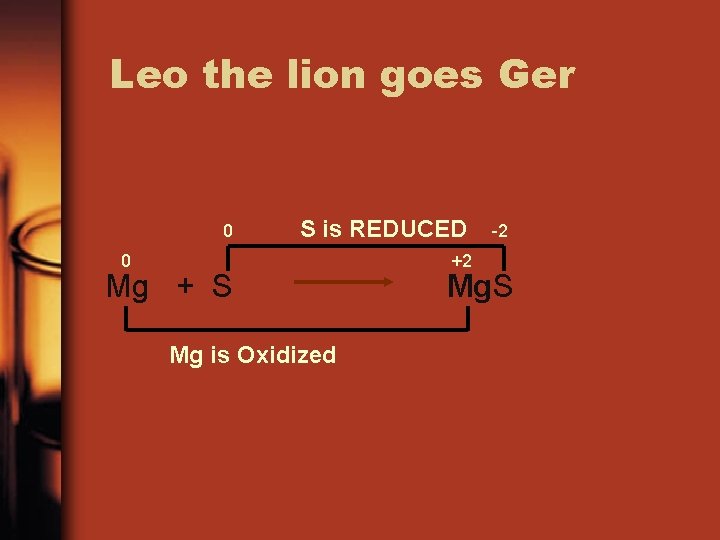
Leo the lion goes Ger 0 S is REDUCED 0 Mg + S Mg is Oxidized +2 -2 Mg. S

Applications of Metal corrosion reactions What is this? ? ?

Applications of Metal corrosion reactions MRE

The idea behind a flameless heater is to use the oxidation of a metal to generate heat. Magnesium metal works better than iron because it rusts much more quickly. To make a flameless heater, magnesium dust is mixed with salt and a little iron dust in a thin, flexible pad about the size of a playing card. To activate the heater, a soldier adds a little water. Within seconds the flameless heater reaches the boiling point and is bubbling and steaming. To heat the meal, the soldier simply inserts the heater and the MRE pouch back in the box that the pouch came in. Ten minutes later, dinner is served!

MRE

Daniels Electrochemical Cell Video
- Slides: 36