Chemical Balancing Rules Know the Symbol Equation showing
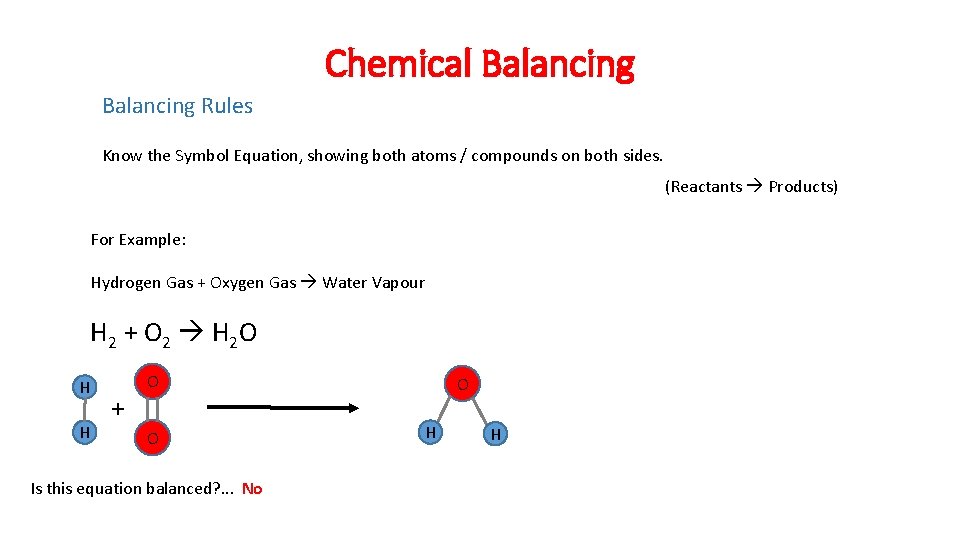
Chemical Balancing Rules Know the Symbol Equation, showing both atoms / compounds on both sides. (Reactants Products) For Example: Hydrogen Gas + Oxygen Gas Water Vapour H 2 + O 2 H 2 O H H + O O Is this equation balanced? . . . No O H H
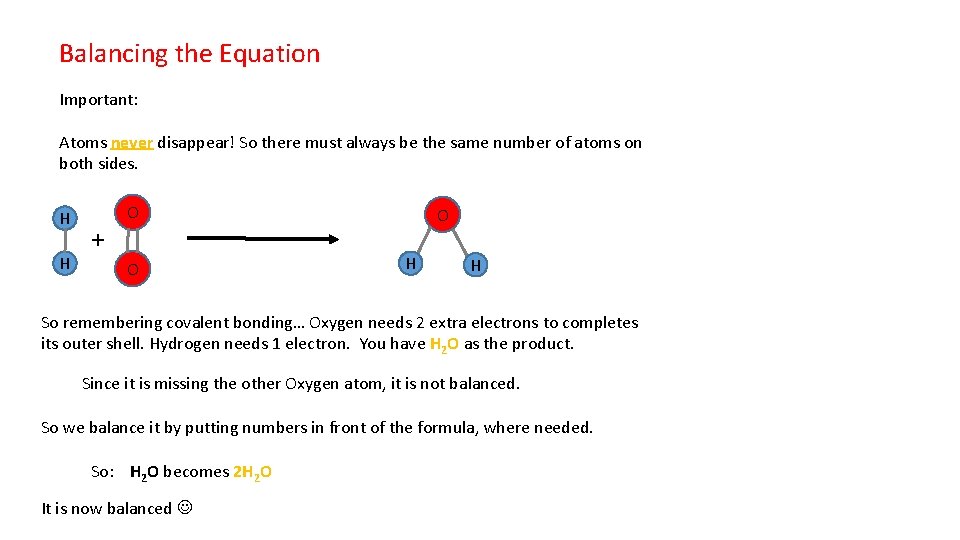
Balancing the Equation Important: Atoms never disappear! So there must always be the same number of atoms on both sides. H H + O O O H H So remembering covalent bonding… Oxygen needs 2 extra electrons to completes its outer shell. Hydrogen needs 1 electron. You have H 2 O as the product. Since it is missing the other Oxygen atom, it is not balanced. So we balance it by putting numbers in front of the formula, where needed. So: H 2 O becomes 2 H 2 O It is now balanced
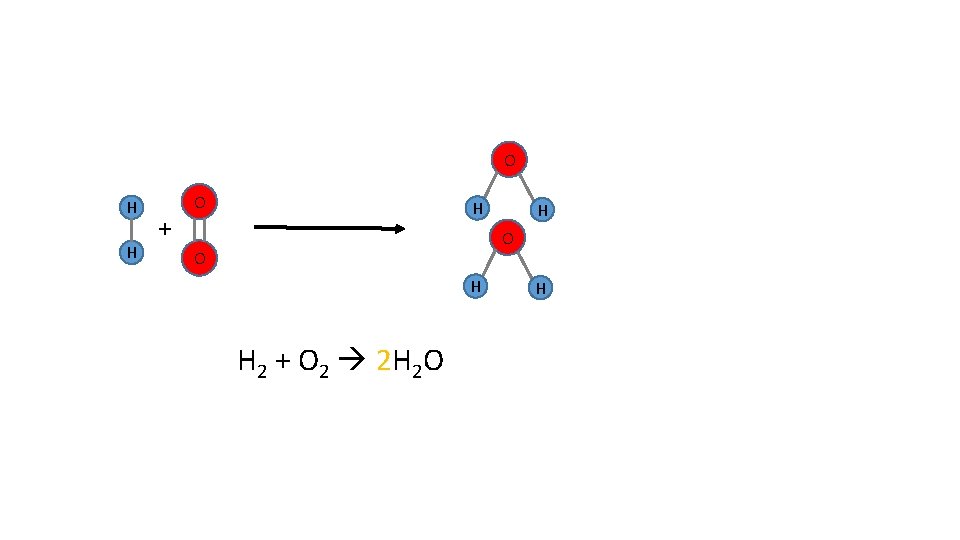
O H H + O H H O O H H 2 + O 2 2 H 2 O H
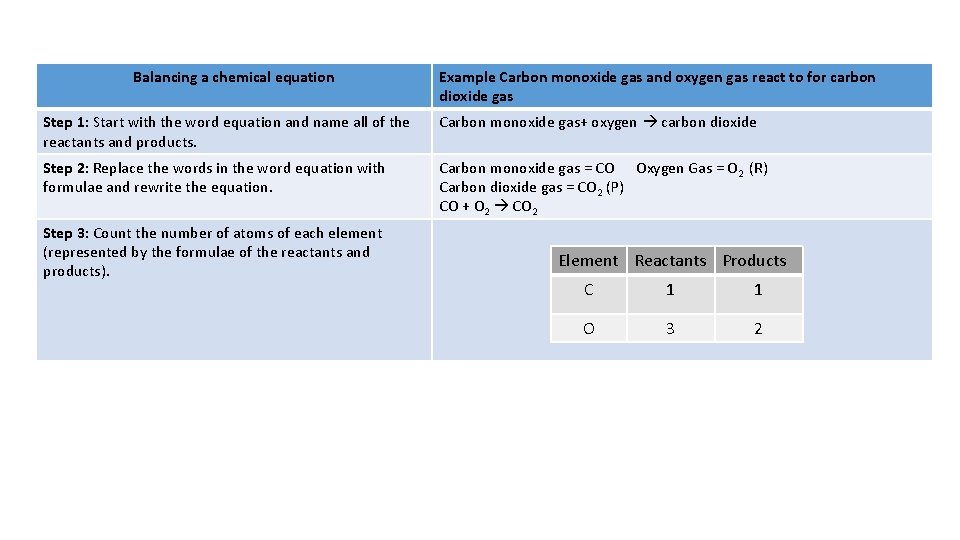
Balancing a chemical equation Example Carbon monoxide gas and oxygen gas react to for carbon dioxide gas Step 1: Start with the word equation and name all of the reactants and products. Carbon monoxide gas+ oxygen carbon dioxide Step 2: Replace the words in the word equation with formulae and rewrite the equation. Carbon monoxide gas = CO Oxygen Gas = O 2 (R) Carbon dioxide gas = CO 2 (P) CO + O 2 CO 2 Step 3: Count the number of atoms of each element (represented by the formulae of the reactants and products). Element Reactants Products C 1 1 O 3 2
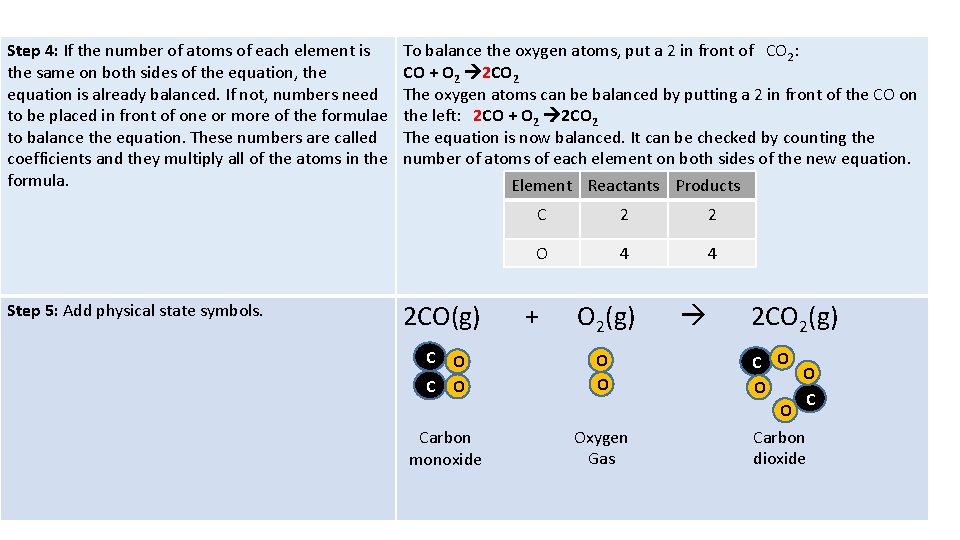
Step 4: If the number of atoms of each element is the same on both sides of the equation, the equation is already balanced. If not, numbers need to be placed in front of one or more of the formulae to balance the equation. These numbers are called coefficients and they multiply all of the atoms in the formula. Step 5: Add physical state symbols. To balance the oxygen atoms, put a 2 in front of CO 2: CO + O 2 2 CO 2 The oxygen atoms can be balanced by putting a 2 in front of the CO on the left: 2 CO + O 2 2 CO 2 The equation is now balanced. It can be checked by counting the number of atoms of each element on both sides of the new equation. Element Reactants Products 2 CO(g) C 2 2 O 4 4 + O 2(g) C O O O Carbon monoxide Oxygen Gas 2 CO 2(g) C O O O Carbon dioxide
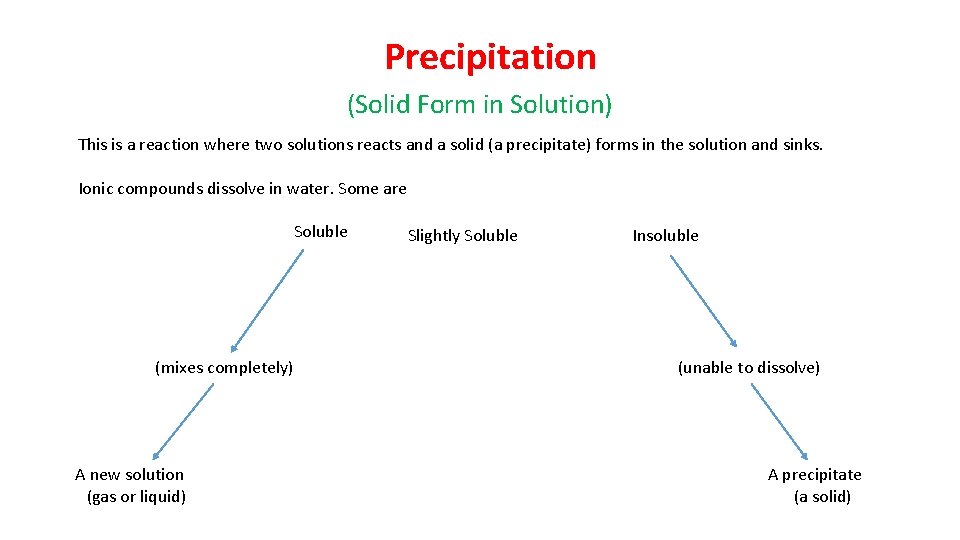
Precipitation (Solid Form in Solution) This is a reaction where two solutions reacts and a solid (a precipitate) forms in the solution and sinks. Ionic compounds dissolve in water. Some are Soluble (mixes completely) A new solution (gas or liquid) Slightly Soluble Insoluble (unable to dissolve) A precipitate (a solid)

Changing Form A precipitation reaction causes the reactants to swap partners in order to create the product. For example: When Silver nitrate solution and Sodium chloride solution are mixed together, the products are Silver chloride (precipitate) and sodium nitrate (aqueous) Word Equation Silver nitrate + Sodium Chloride Silver Chloride + Sodium Nitrate Formula Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. No 3 (aq) All but the Silver chloride are dissolved in water which gives them the symbol (aq) The Silver chloride, being the solid is given the symbol (s).

Prac and Questions: Prac – Will it precipitate? (SQ 10 – p 203) Chapter 5. 3 Questions (1 -6)
- Slides: 8