Chemical and Physical Properties and Changes Matter Matter














































- Slides: 62

Chemical and Physical Properties and Changes

Matter • Matter is everywhere. • Matter is anything that takes up space and has mass. • Matter is constantly experiencing both chemical and physical changes.

Physical Properties and Changes Physical Properties are used to observe or describe matter. How do you observe or describe someone? • Height Let’s describe • Hair. Schrute Color Dwight • Eye Color • Weight • Age • Clothing Color

Physical Properties and Changes When we observe or describe something or someone we use “drivers license” descriptions. When we observe or describe MATTER we use some of the same descriptions.

List as many physical properties as you can…

Physical Properties and Changes Things you observe and can describe about matter: • Color • Size • Smell • Freezing Point • Melting Point • Mass • Shape • Volume • Density

Physical Change • Physical changes occur when matter changes its property but not its chemical nature. • Physical property changes could include a change in: texture, shape, size, color, volume, mass, weight, and density.

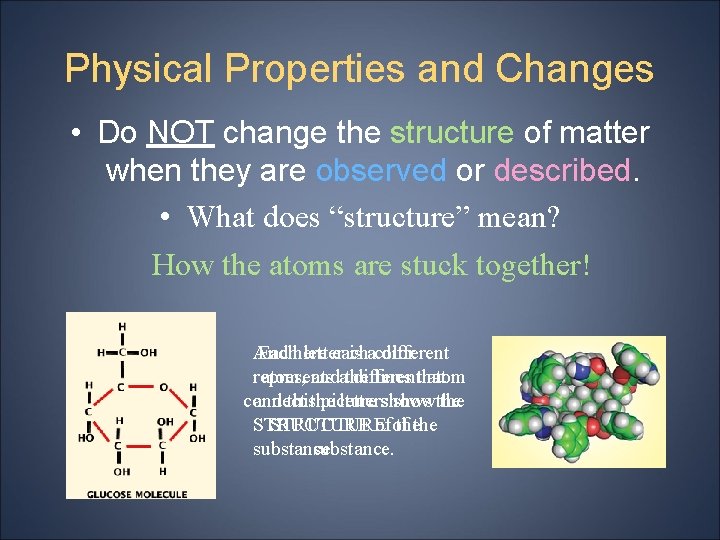
Physical Properties and Changes • Do NOT change the structure of matter when they are observed or described. • What does “structure” mean? How the atoms are stuck together! And Eachhere letter each is acolor different represents atom, andathe different lines that atom connect and thisthe picture lettersshows show the STRUCTURE ofof thethe substance.

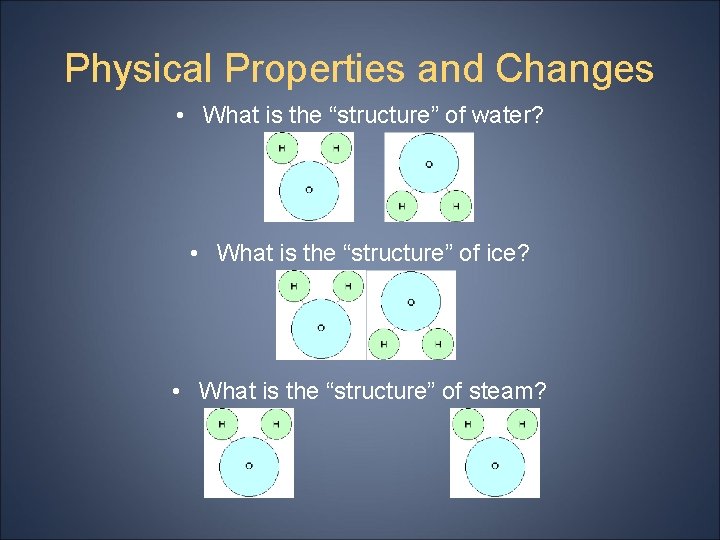
Physical Properties and Changes • What is the “structure” of water? • What is the “structure” of ice? • What is the “structure” of steam?

Physical Properties and Changes • We just observed one of the physical properties of H 2 O! PHASE CHANGE We observed how H 2 O is different in each phase, but the structure of H 2 O never changed!

The difference between Physical Property and Physical change. • The physical property is something that we can measure with out changing the identity of the matter. That includes the materials ability to change but not change what it is • The physical change is the actual process of changing. Remember that no new substance it formed!

Physical Properties and Changes When a physical property (like the phase of water) is altered we called it a PHYSICAL CHANGE In other words: A physical change involves a change in physical properties (shape, size, density, etc. ) It takes a physical change to change water to steam, or ice to liquid. What are some other examples of physical changes?

Physical Properties and Changes • Shape The shape of the wood changed. But is it still a piece of wood?

Physical Properties and Changes • Size

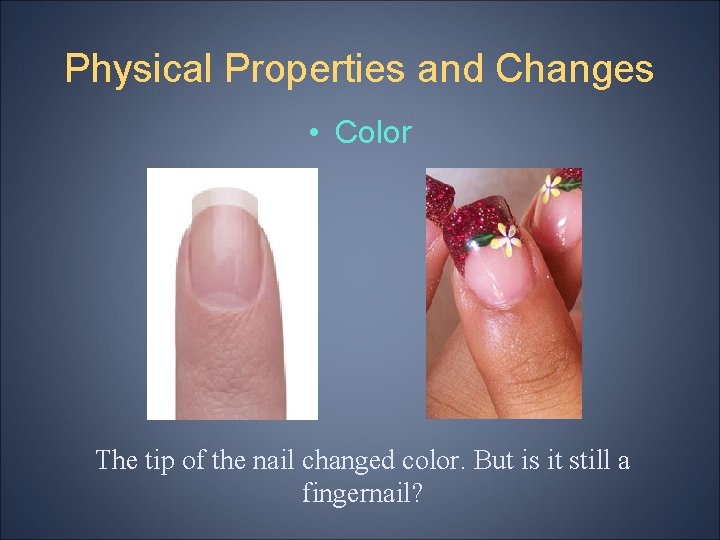
Physical Properties and Changes • Color The tip of the nail changed color. But is it still a fingernail?

Physical Properties and Changes • Melting Point The ice cream melted. But does it still taste like ice cream?

Physical Properties and Changes You can think of Physical Changes like changing the font of a word… Mrs. Hall The “structure” of the words is still the same, even though the physical appearance changed.

Chemical Change • Chemical changes are changes matter undergoes when it becomes new or different matter. • To identify a chemical change look for signs such as color change, bubbling and fizzing, light production, smoke, odor, and presence of heat.

Chemical Properties and Changes • And you can think of Chemical Changes like rearranging the letters of the words… Mrs. Coates Most Acres Cream Toss Re: Mascots Me Toss Cares Most

Chemical Properties and Changes The letters are the same, but we’ve rearranged the structure and made new words! This is what happens in a chemical change! Chemical properties lead the way for chemical change

Chemical Properties and Changes What are some examples of Chemical Properties? • Flammability • Oxidation • Toxicity • Radioactivity • Sensitivity to Light • Reactivity with water

Chemical Properties and Changes • Flammability: – The ability to catch fire!

Chemical Properties and Changes • Oxidation – The ability to react with Oxygen

Chemical Properties and Changes • Toxicity – The ability to be poisonous

Chemical Properties and Changes • Sensitivity to Light – The ability to change when exposed to light

Chemical Properties and Changes • Reactivity with water – Some elements react explosively with water! – Sodium, Potassium, Lithium, and Calcium

Chemical Change • A chemical change occurs when fireworks are used. Fireworks are made of metals such as magnesium and copper. These change chemically as they light up the sky.

Examples of Chemical changes • • Soured milk Alka seltzer Statue of liberty Space shuttle launch

Chemistry & Matter • We can explore the MACROSCOPIC world — what we can see — • to understand the PARTICULATE worlds we cannot see.

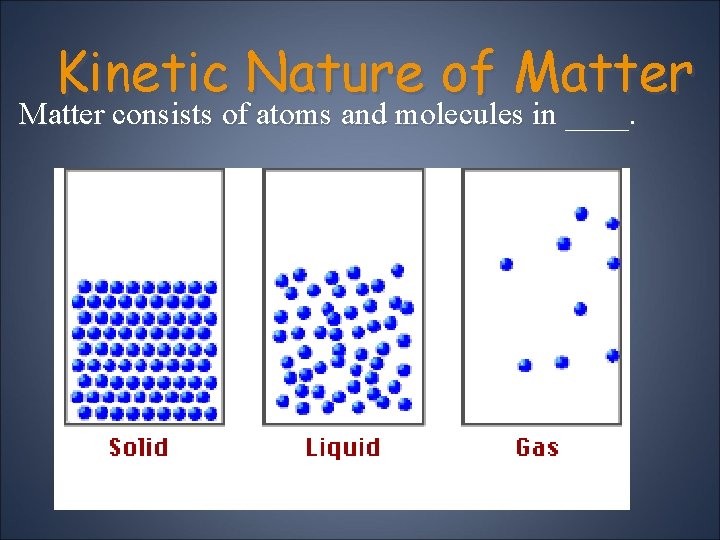
Kinetic Nature of Matter consists of atoms and molecules in ____.

The In-Betweens: Physical or chemical? • Some properties (abilities) can be either physical or chemical • Examples: – Ability to change color – Ability to make a gas

Both of these examples are making a gas. Which one is physical and which is chemical?

Both of these pictures show a color change due to heat: which is physical and which is chemical?

Lets Take the QUIZ… • http: //vital. cs. ohiou. edu/steamwebsite/dow nloads/Change. Lab. swf

1. Is it a chemical or physical change? • Sugar dissolving in tea • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

2. Is it a chemical or physical change? • Logs burning • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

3. Is it a chemical or physical change? • Breaking water up by separating it into hydrogen and oxygen • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

4. Is it a chemical or physical change? • Cutting paper • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

5. Is it a chemical or physical change? • Crushing an aspirin • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

6. Is it a chemical or physical change? • Metal rusting • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

7. Is it a chemical or physical change? • Lighter fluid burning • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

8. Is it a chemical or physical change? • An egg rotting • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

9. Is it a chemical or physical change? • An egg breaking • Chemical Change • Physical Change

OOPS! Did it change size, color, shape (Physical Change)? or Did it become different matter (Chemical Change)?

Correct!

Writing Activity • Write a paragraph about the difference between a chemical and physical change. Give examples of each.