Chemical Analysis Pure and impure In everyday life



















- Slides: 29

Chemical Analysis

• Pure and impure • In everyday life a pure substance is one which has had nothing added to it, like milk. • An impure substance would be one with other things added, like chocolate milk

• In chemistry: • A pure substance consists of a single element or a single compound and nothing else • Impure substances are mixtures and contain other substances

• We can tell the difference by looking at melting and boiling points. Pure substances will have a very sharp melting and boiling point. • For example, pure water freezes at exactly 0°C • Impure substances will tend to melt or boil across a range of temperatures • For example, impure water might begin freezing at 4°C and finish freezing at 0°C

• Questions 1 and 2 on page 181

• Formulations


• Formulations are carefully designed mixtures with many ingredients in very specific amounts. Each of the ingredients has a purpose. • Examples include fuels, paints, medicines, alloys and many more

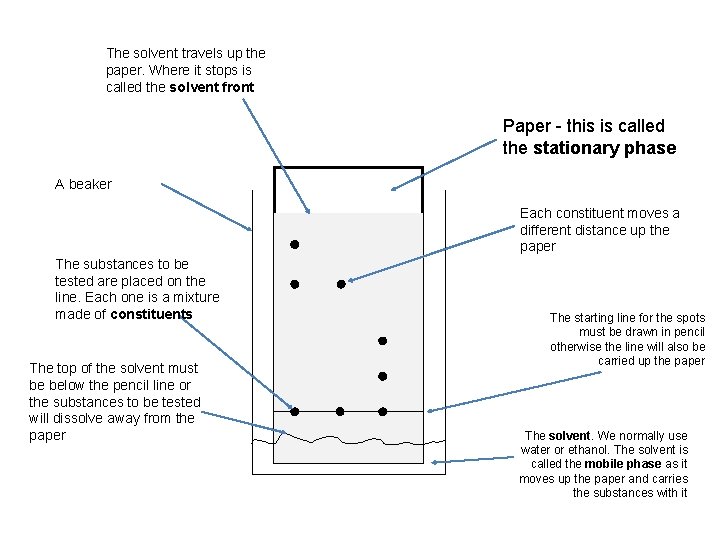
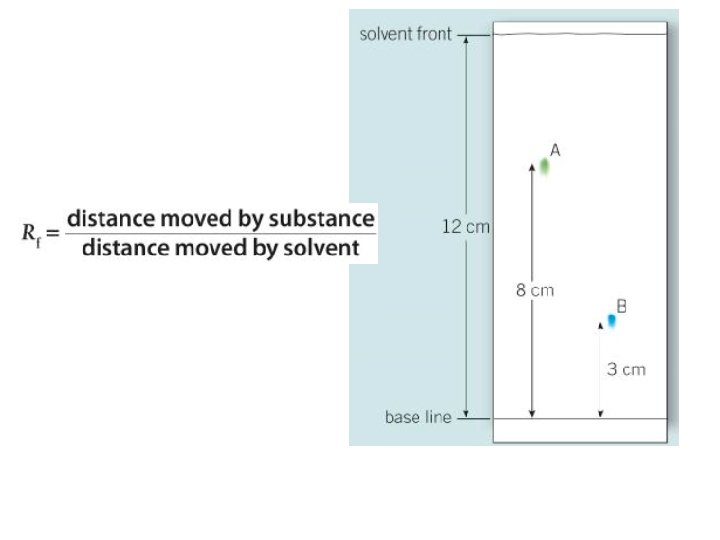
Chromatography

The solvent travels up the paper. Where it stops is called the solvent front Paper - this is called the stationary phase A beaker Each constituent moves a different distance up the paper The substances to be tested are placed on the line. Each one is a mixture made of constituents The top of the solvent must be below the pencil line or the substances to be tested will dissolve away from the paper The starting line for the spots must be drawn in pencil otherwise the line will also be carried up the paper The solvent. We normally use water or ethanol. The solvent is called the mobile phase as it moves up the paper and carries the substances with it

Analysis • In this case, spot 1 and 3 are made of two constituents so are impure. • Spot 2 is made from one constituent so is pure. • Spot 1 has two constituents, one of which also makes up spot 2. • Spot 3 has two constituents, but neither of those are present in spot 1 or 2 • Q 1 -4


• Finish questions

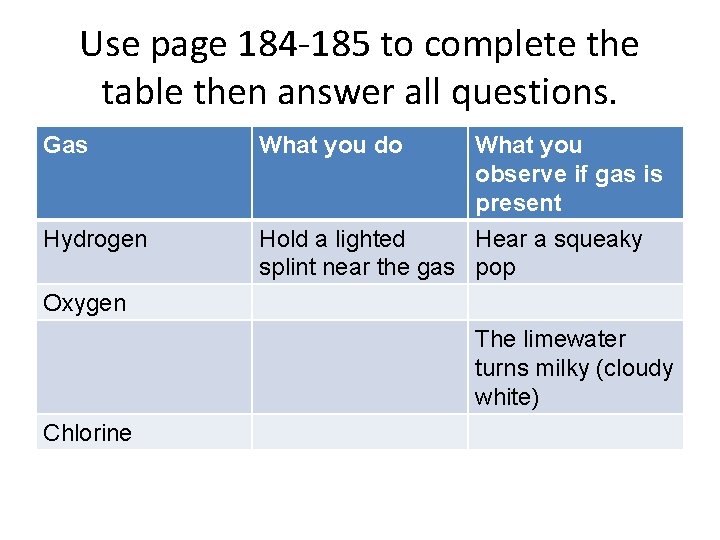
Gas tests • Test for: – Hydrogen – Oxygen – Carbon Dioxide – Chlorine

Use page 184 -185 to complete the table then answer all questions. Gas Hydrogen What you do What you observe if gas is present Hold a lighted Hear a squeaky splint near the gas pop Oxygen The limewater turns milky (cloudy white) Chlorine

Further Analysis – Triple only

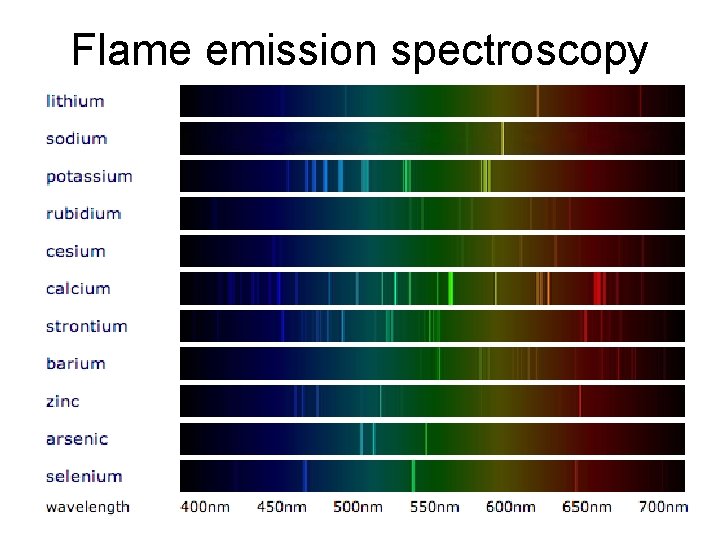
Further Analysis – Triple only • Flame tests: – lithium compounds result in a crimson flame – sodium compounds result in a yellow flame – potassium compounds result in a lilac flame – calcium compounds result in an orange-red flame – copper compounds result in a green flame.

Instrumental Analysis • Instrumental analysis uses complicated scientific equipment to analyse substances • Advantages: – Accurate – Sensitive – Rapid


Flame emission spectroscopy

Tests for ions in solution • Cation tests (positive ions – so metals) • Anion tests (negative ions – non-metals)

Cation tests

Cation tests • Sodium hydroxide solution can be used to identify some metal ions (cations). • Solutions of aluminium, calcium and magnesium ions form white precipitates when sodium hydroxide solution is added but only the aluminium hydroxide precipitate dissolves in excess sodium hydroxide solution. • Solutions of copper(II), iron(II) and iron(III) ions form coloured precipitates when sodium hydroxide solution is added. • Copper(II) forms a blue precipitate, iron(II) a green precipitate and iron(III) a brown precipitate. • Balanced equations for each

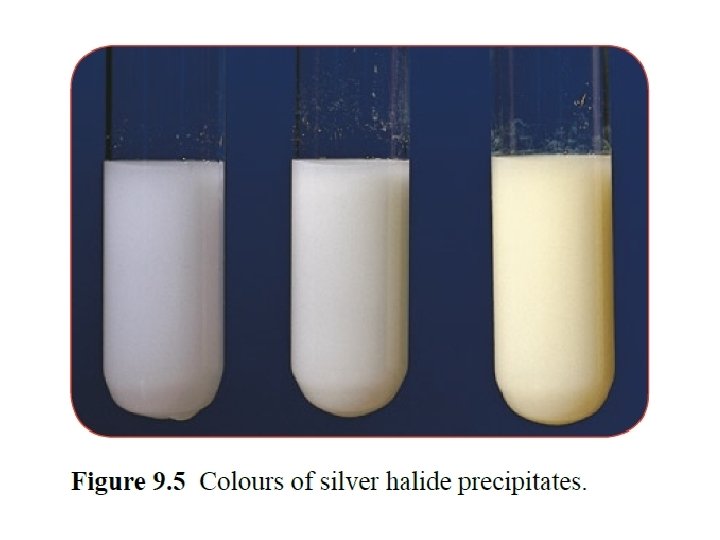
Anion tests • Carbonates react with dilute acids to form carbon dioxide gas. Carbon dioxide can be identified with limewater. • Halide ions in solution produce precipitates with silver nitrate solution in the presence of dilute nitric acid. Silver chloride is white, silver bromide is cream and silver iodide is yellow. • Sulfate ions in solution produce a white precipitate with barium chloride solution in the presence of dilute hydrochloric acid.



• Required prac 7 • Demos

• Conduct a flame test on each of the solutions • Note the colour and therefore the metal involved • Na. OH test: – Put 2 cm 3 of the test solution in a test tube, and add sodium hydroxide solution drop-wise until no further change. – Note the colour of the precipitate and whether it dissolves in excess sodium hydroxide. – Repeat for each test solution in turn and record your results in a table • Chromatography questions • Balanced equation for each Na. OH test • Q 04. 1 04. 5 on 193

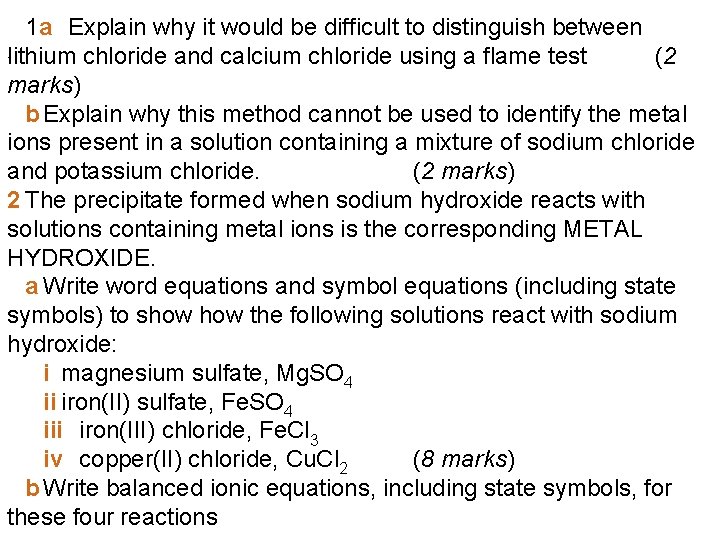
1 a Explain why it would be difficult to distinguish between. lithium chloride and calcium chloride using a flame test (2 marks) b Explain why this method cannot be used to identify the metal ions present in a solution containing a mixture of sodium chloride and potassium chloride. (2 marks) 2 The precipitate formed when sodium hydroxide reacts with solutions containing metal ions is the corresponding METAL HYDROXIDE. a Write word equations and symbol equations (including state symbols) to show the following solutions react with sodium hydroxide: i magnesium sulfate, Mg. SO 4 ii iron(II) sulfate, Fe. SO 4 iii iron(III) chloride, Fe. Cl 3 iv copper(II) chloride, Cu. Cl 2 (8 marks) b Write balanced ionic equations, including state symbols, for these four reactions