Chemical Activation Reactions of Cyclic Alkane and Ether


- Slides: 36

Chemical Activation Reactions of Cyclic Alkane and Ether Ring. Opened Diradicals with O 2: Thermochemistry, Reaction Paths, Kinetics Itsaso Auzmendi Murua, Jason Hudzik Joseph W. Bozzelli 7 th International Conference on Chemical Kinetics, July 10 -14, 2011 Department of Chemical Engineering

Introduction • Cyclic Aliphatic Hydrocarbons are major components in modern fuels: - Present in reactants: § Commertial jet fuel contains: 26% cycloalkanes and alkylcycloalkanes § Commercial diesel fuel (up to 40%) and gasoline (up to 3%) - Produced during the gas-phase processes • During combustion or pyrolisis processes, cycloalkanes can lead to formation of: - Toxic compounds or soot precursors such as benzene (via dehydrogenation) - Linear unsaturated species such as acrolein (via ring opening) • 3 to 6 member cyclic ethers are formed at early times by alkyl radical reactions with dioxygen in combustion and pre-combustion processes that occur at moderate T.

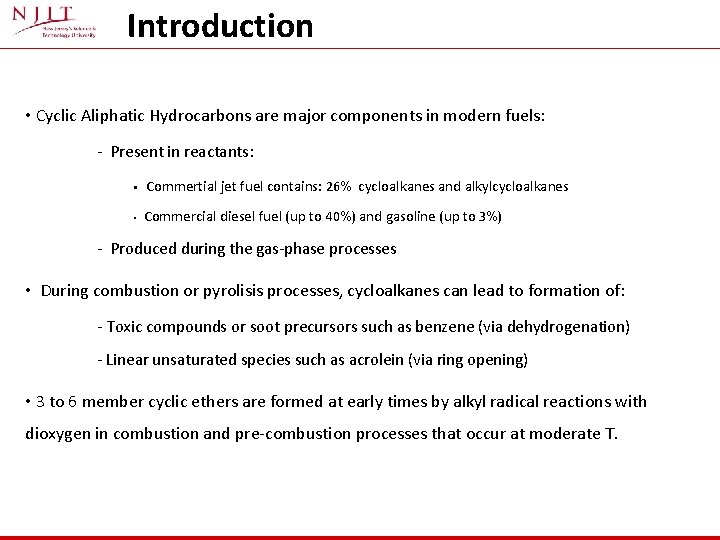
Introduction – s-butane oxidation Formation of Cyclic Ethers in Alkyl Radical Oxidation R. + O 2 => ROO. Hydrogen atom transfer then Cyclic ether formed with OH elimination

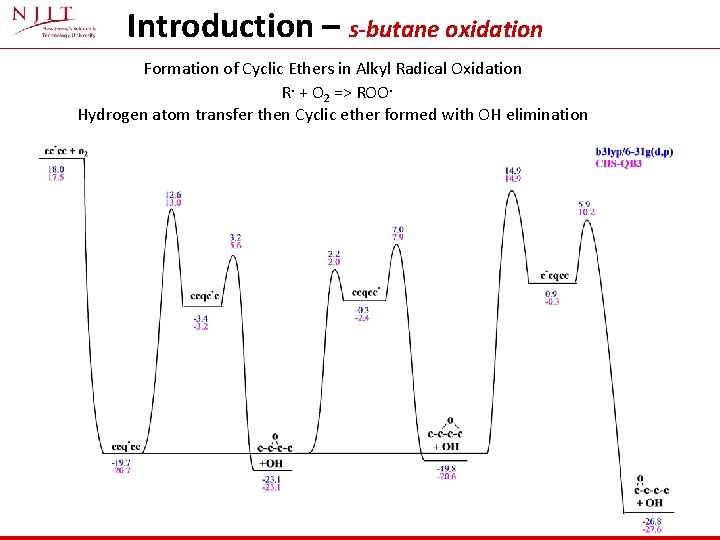
Introduction – s- and t- isooctane oxidation Formation of Cyclic Ethers In Isooctane Radical + O 2 Reactions

Introduction • Initial unimolecular dissociation reactions of cyclic alkanes and ethers in combustion systems are ring opening to form a di-alkyl radical. • Release of ring strain in small ( 3 to 5 member ring) and bicyclic molecules reduces the bond energy needed for bond cleavage - ring opening – Diradical Formation. • The initial ring opened di-radical or the peroxy – alkyl di-radical can undergo triplet – singlet conversion by: - Electronic state crossing - Collisions of the di-alkyl radical with the bath gas - Chemical activation reaction of one radical site via association with 3 O 2

Introduction • This study is an attempt to determine the importance of the diradicals reacting with dioxygen. • Quantum chemical calculations for thermochemical properties. • Statistical rate theory for the T and P dependence of the rate coefficient • Systems Studied : - Cyclic Alkanes : y(ccc), y(cccc) and y(ccccc) - Cyclic Ethers : y(cco), y(ccco) and y(cccco) - TCD (C 10 H 16) Tri-cyclo Decane

Thermochemical Properties • Use of computational chemistry → calculate for radicals and molecules: - Heats of formation - Entropies - Heat capacities • Heat of formartion from Isodesmic work reactions: + + cc. ccccc. Ave σ (*) Sirjean, B. , et al. . J. Phys. Chem. A 2006, 110, 12693. 67. 51 0. 29 Literature (*) 67. 54 67. 66 67. 58 67. 83 67. 65 Group Additivity 67. 52 67. 80 67. 55 67. 94 67. 70 From Bond Energies 67. 18 67. 38 67. 02 67. 11 67. 17 Average methods B 1 B 95 6 -31 g(d, p) c. ccc. c. cc = cccc. + ccccc = cccc. BMK 6 -31 g(d, p) Work Reactions B 3 LYP 6 -31 g(d, p) ΔHf (kcal/mol) 67. 51 67. 74 67. 43

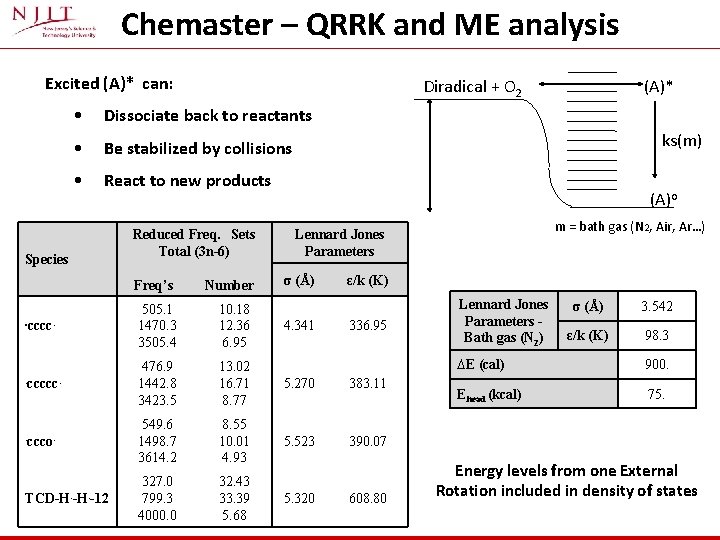
Rate Constants Association and addition reactions are treated as: § Chemical activation reactions with: o Quantum Rice Ramsperger Kassel analysis for k(E) o Master Equation for fall-off (pressure dependant reactions) o Steady State Analysis for Activated Species Input file for Chemaster: • Thermochemical information on reaction paths • Temperature and pressures desired for study • Frequencies of the species involved in the reactions • High Pressure Rate Constants • Lennard Jones Collision Parameters of reactants and the bath gas • ΔEdown and ΔEaverage for the determination of k(E)

Chemaster – QRRK and ME analysis Excited (A)* can: Diradical + O 2 • Dissociate back to reactants • Be stabilized by collisions • React to new products Species Reduced Freq. Sets Total (3 n-6) (A)* ks(m) (A)o m = bath gas (N 2, Air, Ar…) Lennard Jones Parameters σ (Å) ε/k (K) Freq’s Number . cccc. 505. 1 1470. 3 3505. 4 10. 18 12. 36 6. 95 . ccccc. 476. 9 1442. 8 3423. 5 13. 02 16. 71 8. 77 5. 270 383. 11 . ccco. 549. 6 1498. 7 3614. 2 8. 55 10. 01 4. 93 5. 523 390. 07 TCD-H. -12 327. 0 799. 3 4000. 0 32. 43 33. 39 5. 68 5. 320 4. 341 336. 95 608. 80 Lennard Jones Parameters Bath gas (N 2) σ (Å) 3. 542 ε/k (K) 98. 3 ∆E (cal) 900. Ehead (kcal) 75. Energy levels from one External Rotation included in density of states

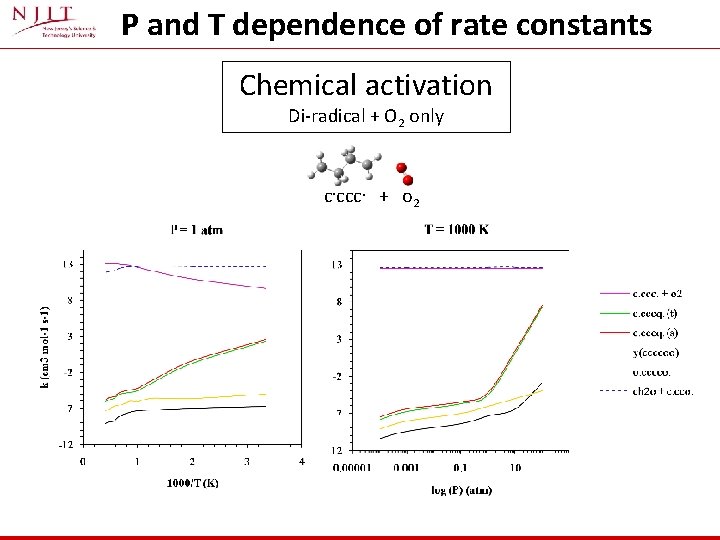
P and T dependence of rate constants Chemical activation Di-radical + O 2 only c. ccc. + o 2

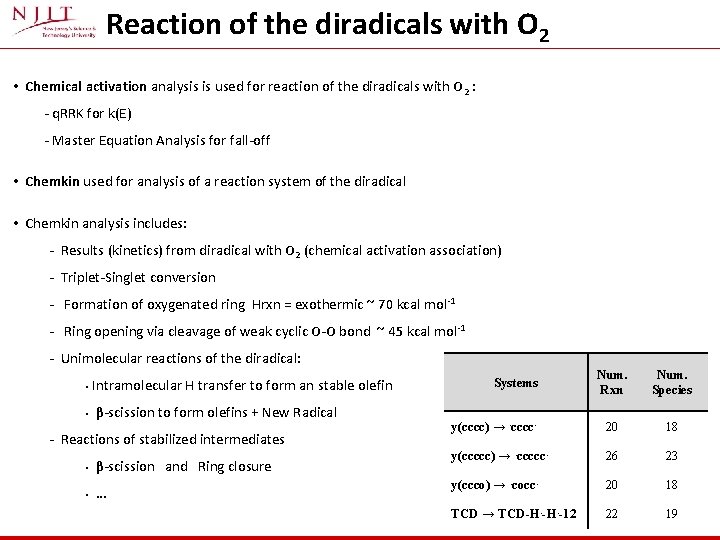
Reaction of the diradicals with O 2 • Chemical activation analysis is used for reaction of the diradicals with O 2 : - q. RRK for k(E) - Master Equation Analysis for fall-off • Chemkin used for analysis of a reaction system of the diradical • Chemkin analysis includes: - Results (kinetics) from diradical with O 2 (chemical activation association) - Triplet-Singlet conversion - Formation of oxygenated ring Hrxn = exothermic ~ 70 kcal mol-1 - Ring opening via cleavage of weak cyclic O-O bond ~ 45 kcal mol-1 - Unimolecular reactions of the diradical: § § Intramolecular H transfer to form an stable olefin β-scission to form olefins + New Radical - Reactions of stabilized intermediates § β-scission and Ring closure § … Num. Rxn Num. Species y(cccc) →. cccc. 20 18 y(ccccc) →. ccccc. 26 23 y(ccco) →. cocc. 20 18 TCD → TCD-H. -12 22 19 Systems

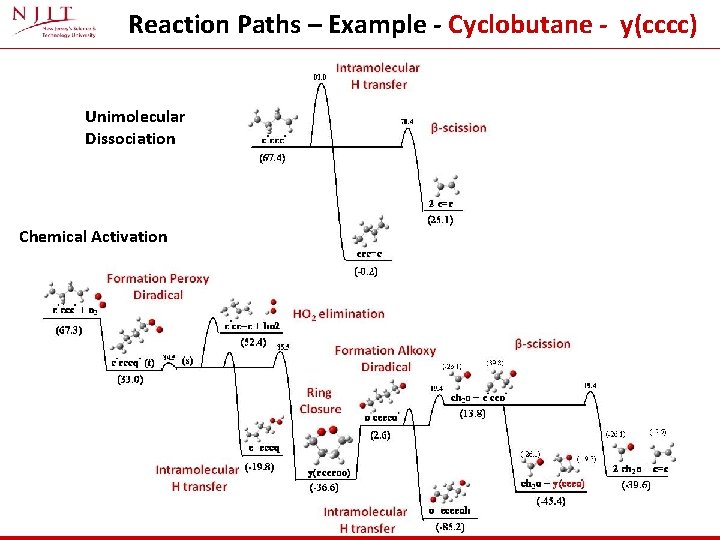
Reaction Paths – Example - Cyclobutane - y(cccc) Unimolecular Dissociation Chemical Activation

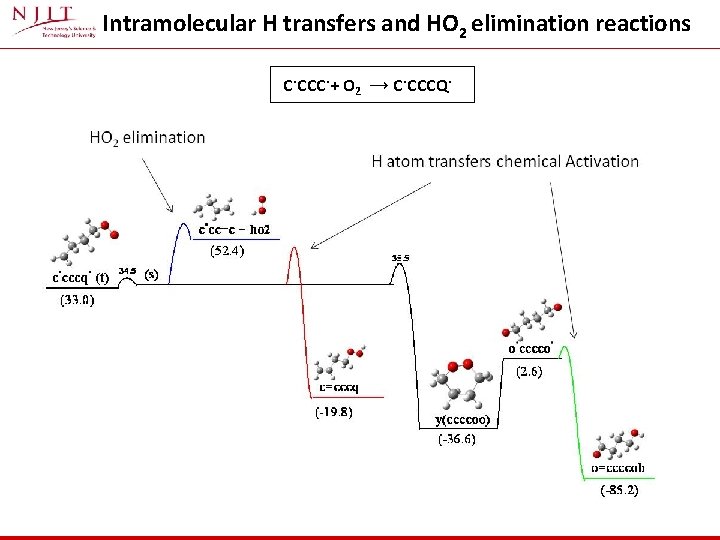
Intramolecular H transfers and HO 2 elimination reactions C. CCC. + O 2 → C. CCCQ.

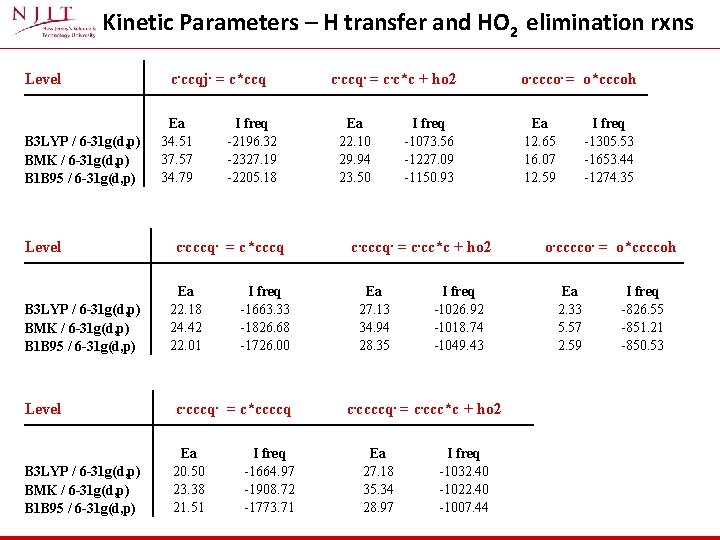
Kinetic Parameters – H transfer and HO 2 elimination rxns Level B 3 LYP / 6 -31 g(d, p) BMK / 6 -31 g(d, p) B 1 B 95 / 6 -31 g(d, p) c. ccqj. = c*ccq Ea 34. 51 37. 57 34. 79 I freq -2196. 32 -2327. 19 -2205. 18 c. cccq. = c*cccq Ea 22. 18 24. 42 22. 01 I freq -1663. 33 -1826. 68 -1726. 00 Level c. cccq. = c*ccccq B 3 LYP / 6 -31 g(d, p) BMK / 6 -31 g(d, p) B 1 B 95 / 6 -31 g(d, p) Ea 20. 50 23. 38 21. 51 I freq -1664. 97 -1908. 72 -1773. 71 c. ccq. = c. c*c + ho 2 Ea 22. 10 29. 94 23. 50 I freq -1073. 56 -1227. 09 -1150. 93 c. cccq. = c. cc*c + ho 2 Ea 27. 13 34. 94 28. 35 I freq -1026. 92 -1018. 74 -1049. 43 c. ccccq. = c. ccc*c + ho 2 Ea 27. 18 35. 34 28. 97 I freq -1032. 40 -1022. 40 -1007. 44 o. ccco. = o*cccoh Ea 12. 65 16. 07 12. 59 I freq -1305. 53 -1653. 44 -1274. 35 o. cccco. = o*ccccoh Ea 2. 33 5. 57 2. 59 I freq -826. 55 -851. 21 -850. 53

CHEMKIN MODELING RESULTS

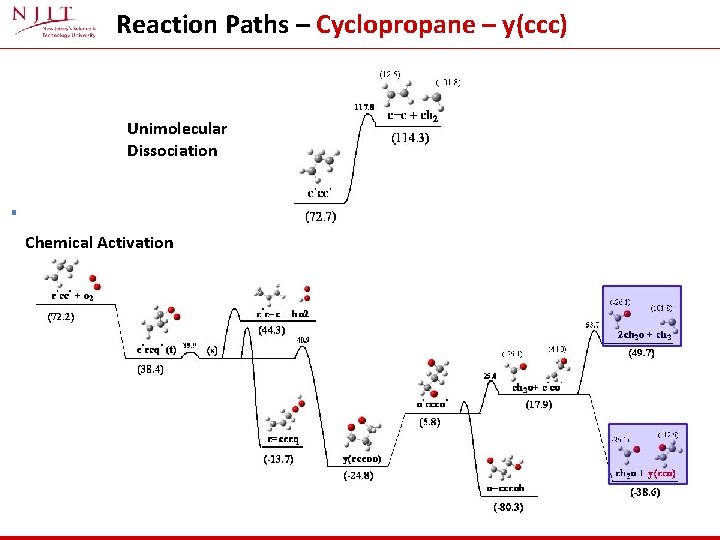
Reaction Paths – Cyclopropane – y(ccc) Unimolecular Dissociation Chemical Activation

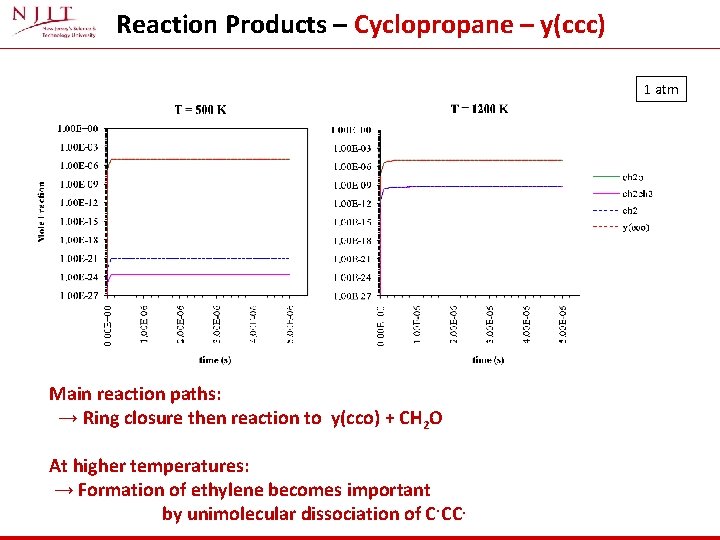
Reaction Products – Cyclopropane – y(ccc) 1 atm Main reaction paths: → Ring closure then reaction to y(cco) + CH 2 O At higher temperatures: → Formation of ethylene becomes important by unimolecular dissociation of C. CC.

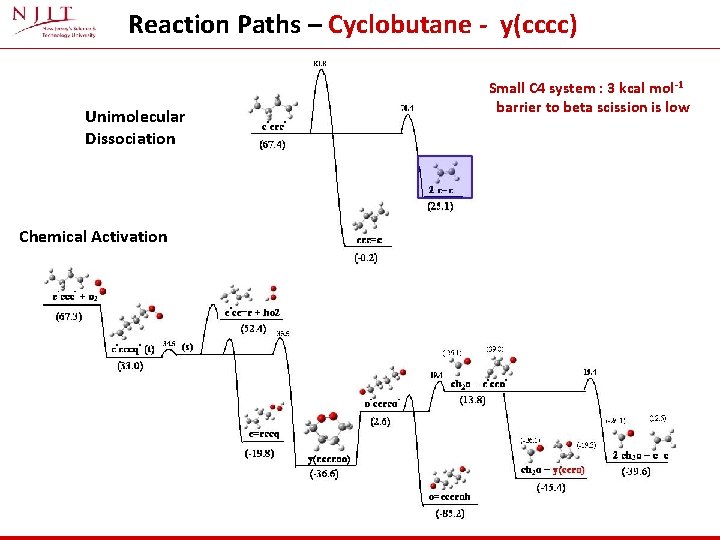
Reaction Paths – Cyclobutane - y(cccc) Unimolecular Dissociation Chemical Activation Small C 4 system : 3 kcal mol-1 barrier to beta scission is low

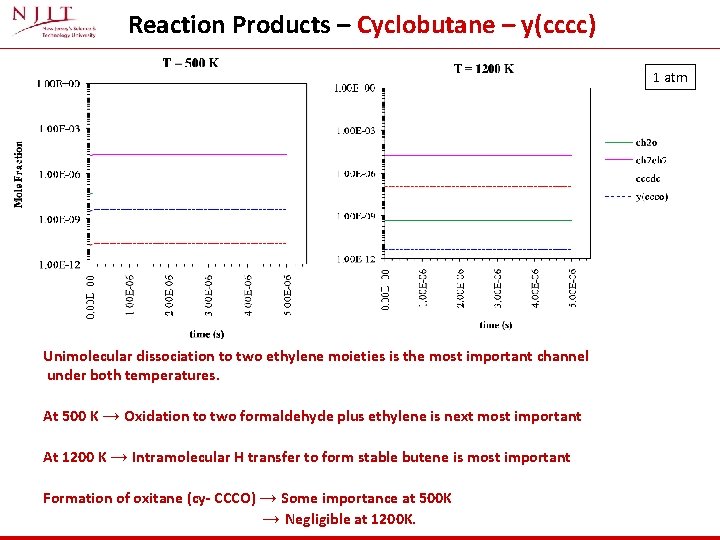
Reaction Products – Cyclobutane – y(cccc) 1 atm Unimolecular dissociation to two ethylene moieties is the most important channel under both temperatures. At 500 K → Oxidation to two formaldehyde plus ethylene is next most important At 1200 K → Intramolecular H transfer to form stable butene is most important Formation of oxitane (cy- CCCO) → Some importance at 500 K → Negligible at 1200 K.

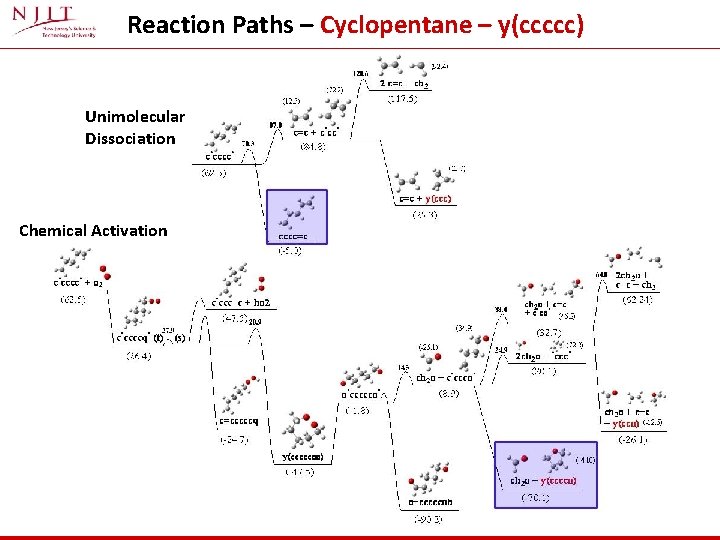
Reaction Paths – Cyclopentane – y(ccccc) Unimolecular Dissociation Chemical Activation

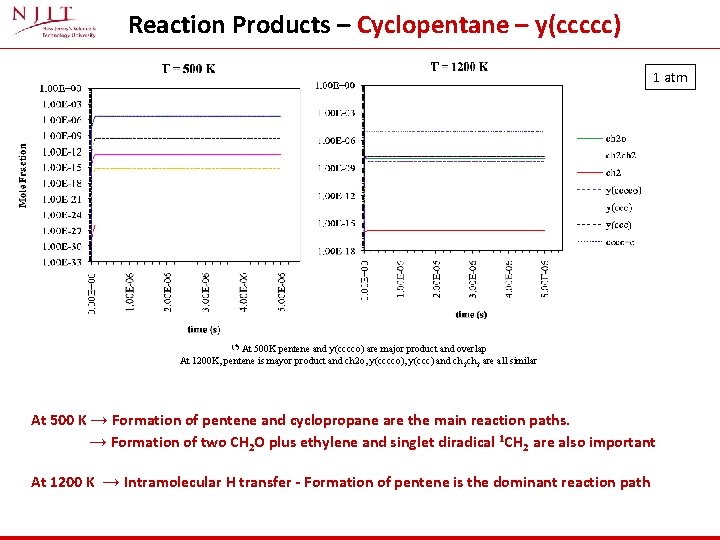
Reaction Products – Cyclopentane – y(ccccc) 1 atm (*) At 500 K pentene and y(cccco) are major product and overlap At 1200 K, pentene is mayor product and ch 2 o, y(cccco), y(ccc) and ch 2 are all similar At 500 K → Formation of pentene and cyclopropane are the main reaction paths. → Formation of two CH 2 O plus ethylene and singlet diradical 1 CH 2 are also important At 1200 K → Intramolecular H transfer - Formation of pentene is the dominant reaction path

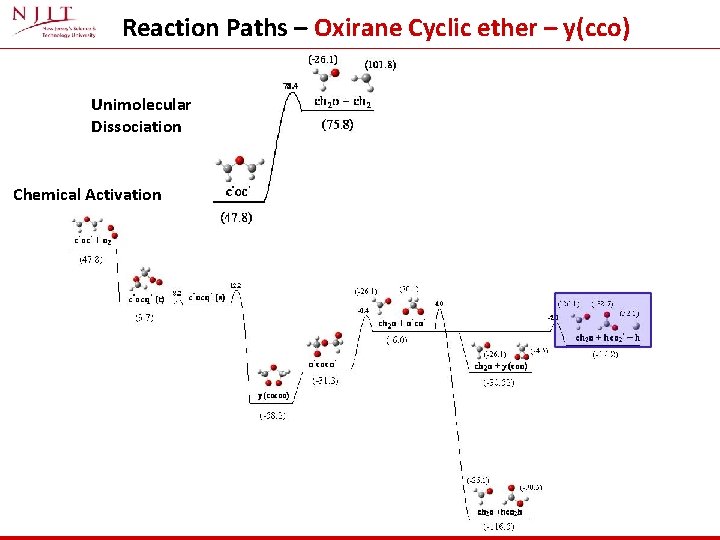
Reaction Paths – Oxirane Cyclic ether – y(cco) Unimolecular Dissociation Chemical Activation

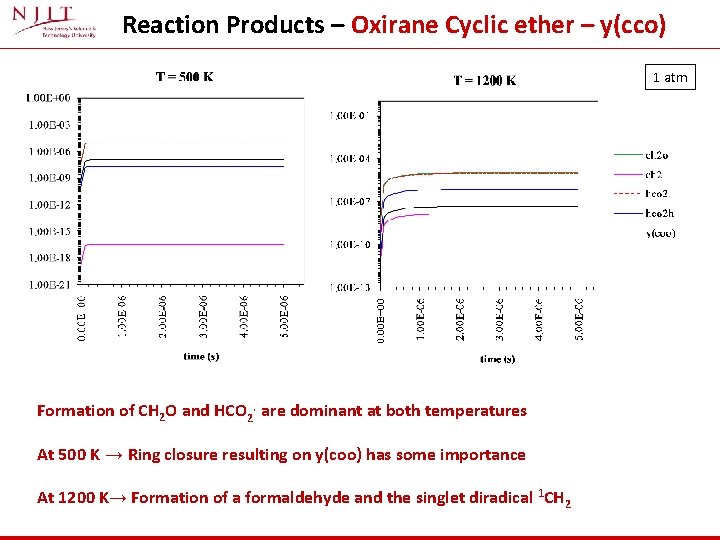
Reaction Products – Oxirane Cyclic ether – y(cco) 1 atm Formation of CH 2 O and HCO 2. are dominant at both temperatures At 500 K → Ring closure resulting on y(coo) has some importance At 1200 K→ Formation of a formaldehyde and the singlet diradical 1 CH 2

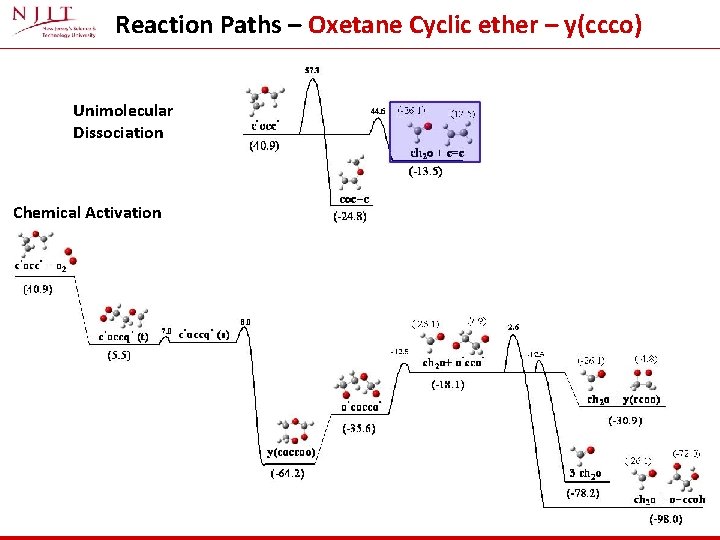
Reaction Paths – Oxetane Cyclic ether – y(ccco) Unimolecular Dissociation Chemical Activation

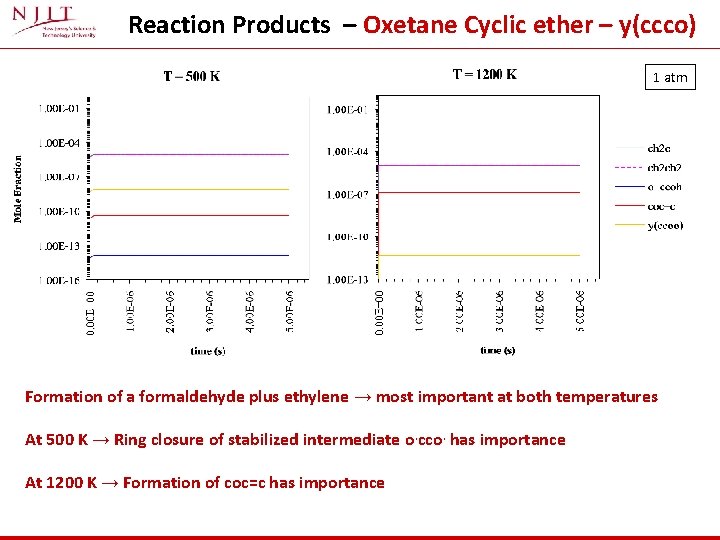
Reaction Products – Oxetane Cyclic ether – y(ccco) 1 atm Formation of a formaldehyde plus ethylene → most important at both temperatures At 500 K → Ring closure of stabilized intermediate o. cco. has importance At 1200 K → Formation of coc=c has importance

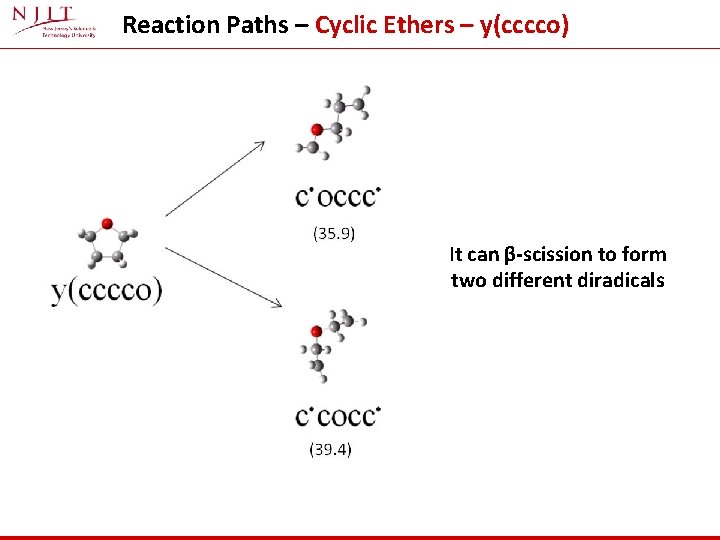
Reaction Paths – Cyclic Ethers – y(cccco) It can β-scission to form two different diradicals

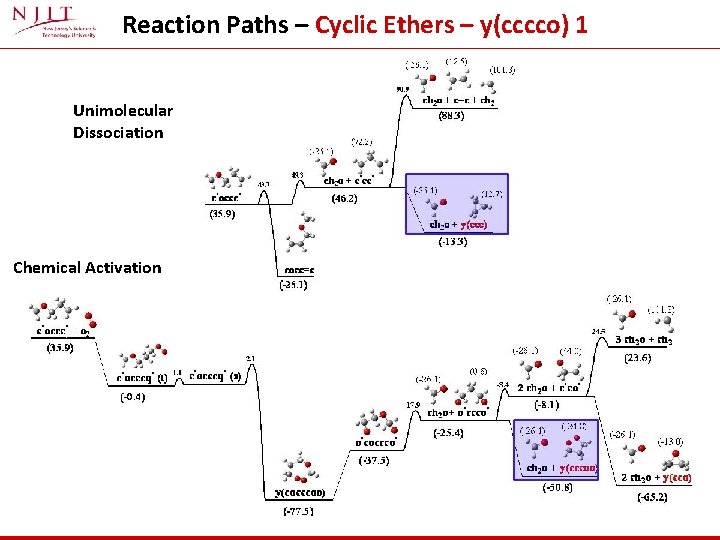
Reaction Paths – Cyclic Ethers – y(cccco) 1 Unimolecular Dissociation Chemical Activation

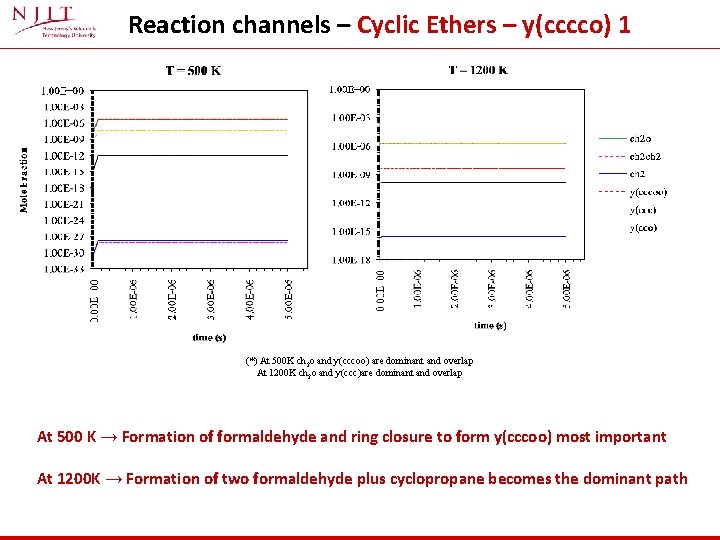
Reaction channels – Cyclic Ethers – y(cccco) 1 (*) At 500 K ch 2 o and y(cccoo) are dominant and overlap At 1200 K ch 2 o and y(ccc)are dominant and overlap At 500 K → Formation of formaldehyde and ring closure to form y(cccoo) most important At 1200 K → Formation of two formaldehyde plus cyclopropane becomes the dominant path

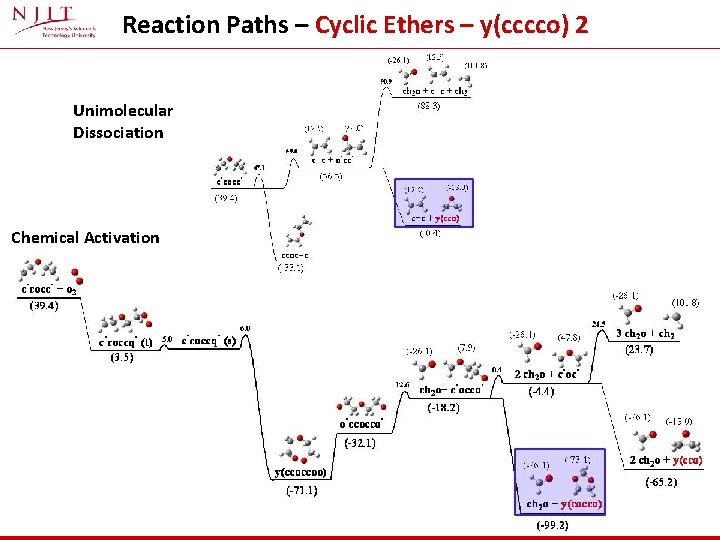
Reaction Paths – Cyclic Ethers – y(cccco) 2 Unimolecular Dissociation Chemical Activation

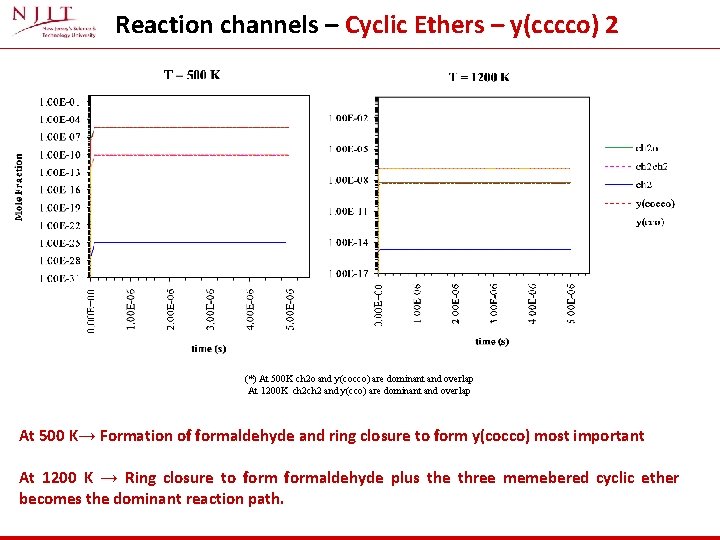
Reaction channels – Cyclic Ethers – y(cccco) 2 (*) At 500 K ch 2 o and y(cocco) are dominant and overlap At 1200 K ch 2 and y(cco) are dominant and overlap At 500 K→ Formation of formaldehyde and ring closure to form y(cocco) most important At 1200 K → Ring closure to formaldehyde plus the three memebered cyclic ether becomes the dominant reaction path.

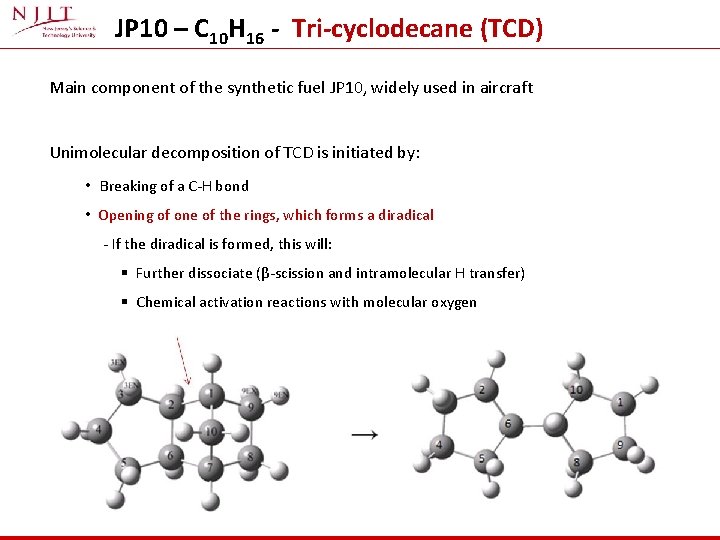
JP 10 – C 10 H 16 - Tri-cyclodecane (TCD) Main component of the synthetic fuel JP 10, widely used in aircraft Unimolecular decomposition of TCD is initiated by: • Breaking of a C-H bond • Opening of one of the rings, which forms a diradical - If the diradical is formed, this will: § Further dissociate (β-scission and intramolecular H transfer) § Chemical activation reactions with molecular oxygen

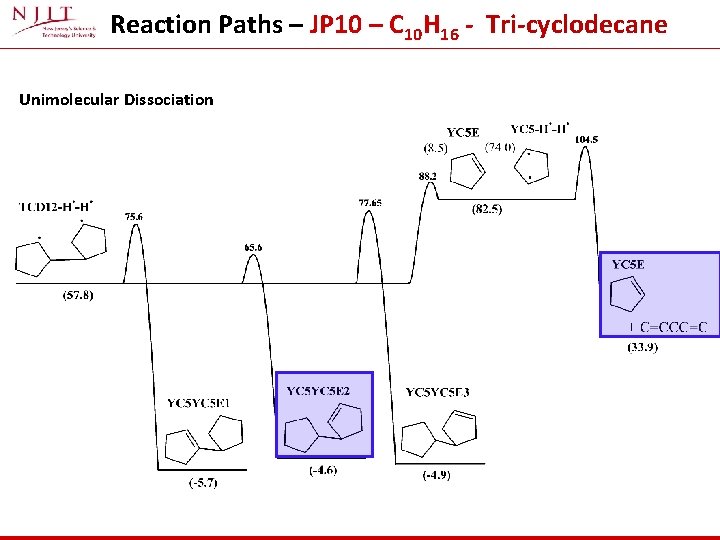
Reaction Paths – JP 10 – C 10 H 16 - Tri-cyclodecane Unimolecular Dissociation

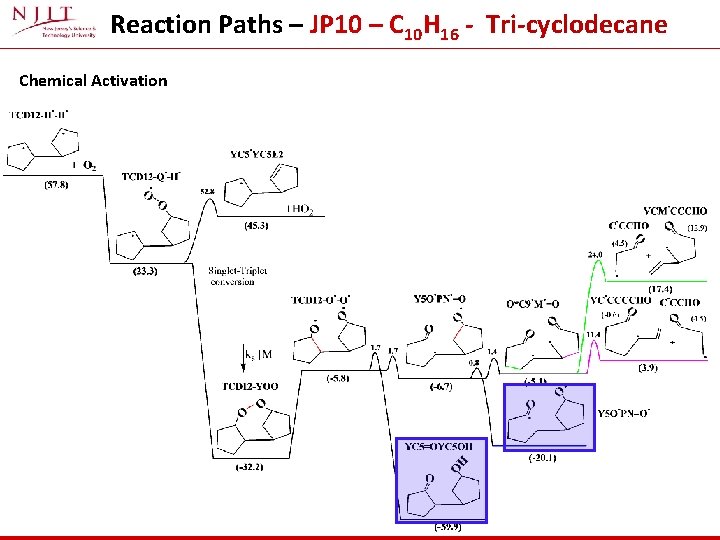
Reaction Paths – JP 10 – C 10 H 16 - Tri-cyclodecane Chemical Activation

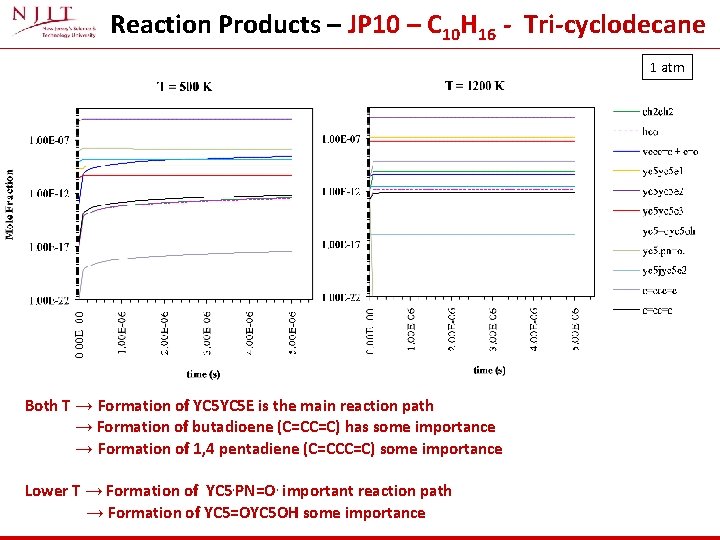
Reaction Products – JP 10 – C 10 H 16 - Tri-cyclodecane 1 atm Both T → Formation of YC 5 E is the main reaction path → Formation of butadioene (C=CC=C) has some importance → Formation of 1, 4 pentadiene (C=CCC=C) some importance Lower T → Formation of YC 5. PN=O. important reaction path → Formation of YC 5=OYC 5 OH some importance

Conclusions • Reformation of cycle → fast function of Ring-Opening → Further reactions • Most ring opening occurs at high temperature → β-scission • β-scission and intramolecular H transfer reactions with low barriers exist → these dominant C. CCC. → 2 C 2 H 4 O. CCCCO. → O=CCCCOH Ea = 3. 0 kcal mol-1 Ea = 2. 3 kcal mol-1 • Where β-scission and intramolecular H transfer reactions are typical (Ea ~ 14 -20 kcal mol-1) → Reactions with O 2 become important at low T • Ring closure from chemical activation intermediate species

Future Work • Further study of intramolecular H transfers for diradicals • Development of unimolecular and chemical activation kinetics for TCD ACKNOWLEDGMENTS • Naval Office of Research • Basque Government