CHEME 7130 Process Modeling Lecture 5 1 Lecture


- Slides: 44

CHEM-E 7130 Process Modeling Lecture 5 1

Lecture 5 outline • When accurate mass transfer models should be used? • Origins of diffusion from molecular collisions (Maxwell-Stefan formulation) including non-conventional driving forces • Matrices, basic operations and matrix functions • Solution of diffusion model with boundary conditions (film model) 2

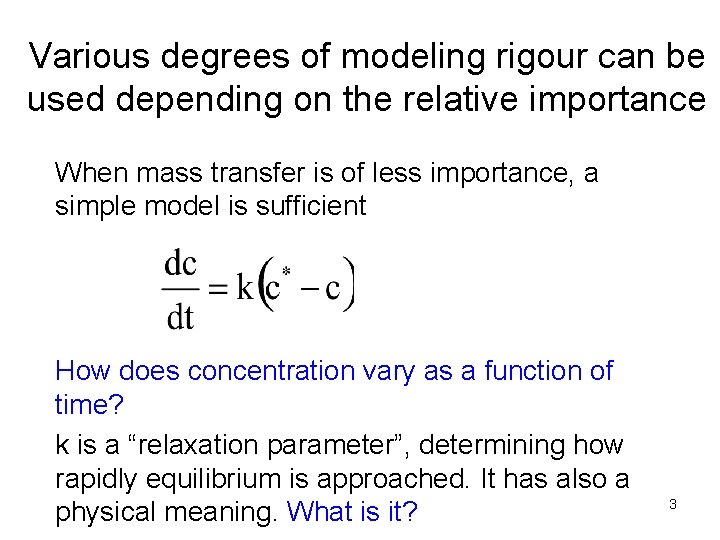
Various degrees of modeling rigour can be used depending on the relative importance When mass transfer is of less importance, a simple model is sufficient How does concentration vary as a function of time? k is a “relaxation parameter”, determining how rapidly equilibrium is approached. It has also a physical meaning. What is it? 3

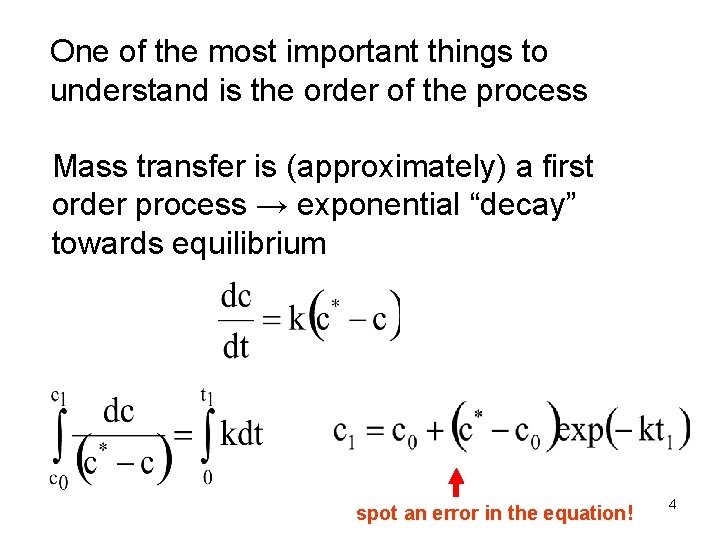
One of the most important things to understand is the order of the process Mass transfer is (approximately) a first order process → exponential “decay” towards equilibrium spot an error in the equation! 4

This level is reasonable in preliminary analysis and estimation Why to use more complicated models? • Specify control volume for material balances correctly • Mechanistic model for mass transfer coefficient and driving forces (extrapolation!) • Understanding the physics of diffusion 5

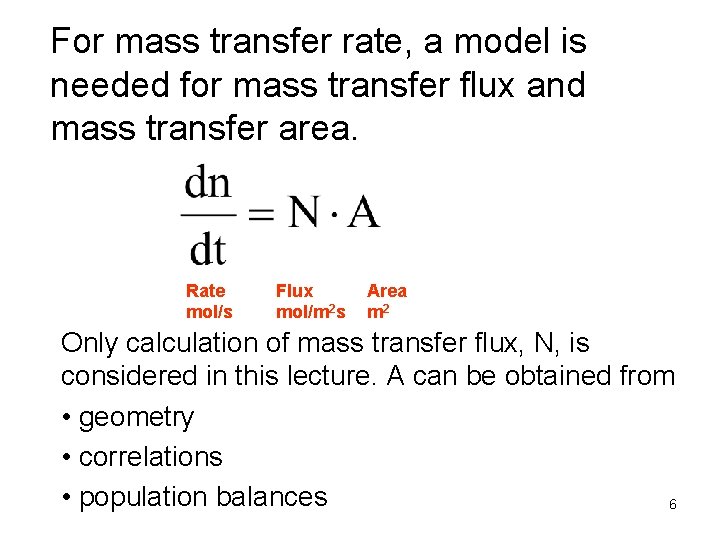
For mass transfer rate, a model is needed for mass transfer flux and mass transfer area. Rate mol/s Flux mol/m 2 s Area m 2 Only calculation of mass transfer flux, N, is considered in this lecture. A can be obtained from • geometry • correlations • population balances 6

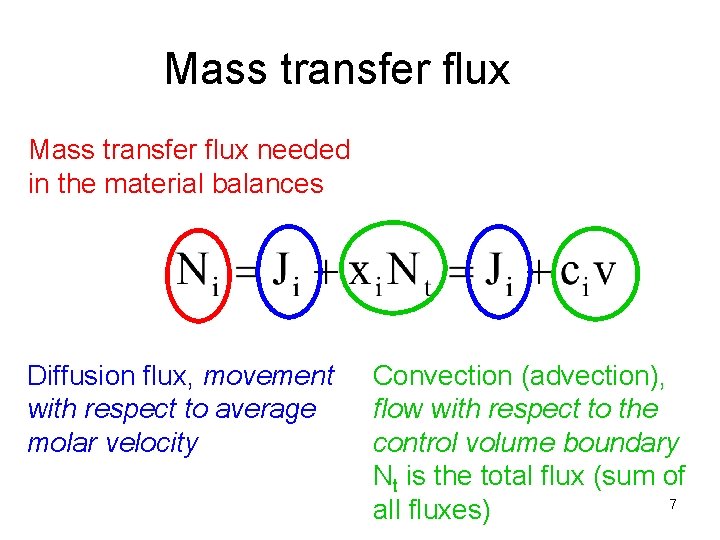
Mass transfer flux needed in the material balances Diffusion flux, movement with respect to average molar velocity Convection (advection), flow with respect to the control volume boundary Nt is the total flux (sum of 7 all fluxes)

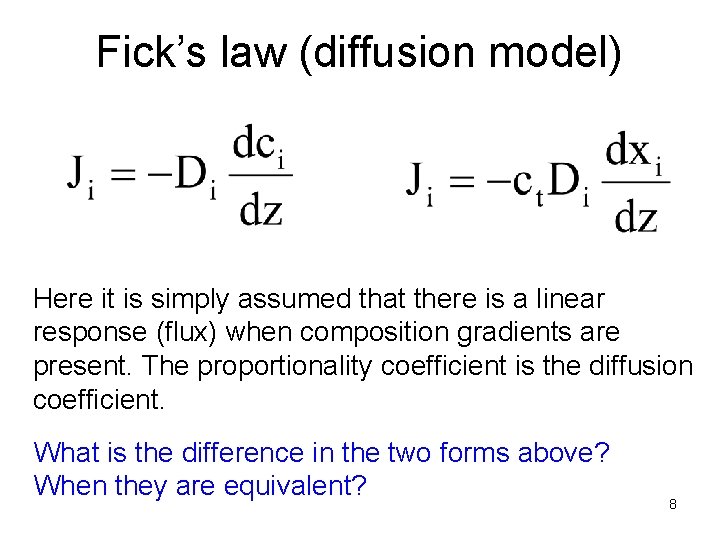
Fick’s law (diffusion model) Here it is simply assumed that there is a linear response (flux) when composition gradients are present. The proportionality coefficient is the diffusion coefficient. What is the difference in the two forms above? When they are equivalent? 8

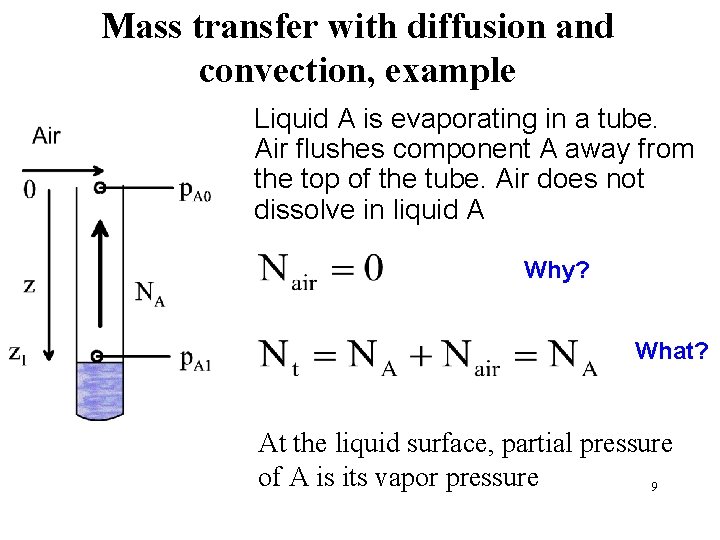
Mass transfer with diffusion and convection, example Liquid A is evaporating in a tube. Air flushes component A away from the top of the tube. Air does not dissolve in liquid A Why? What? At the liquid surface, partial pressure of A is its vapor pressure 9

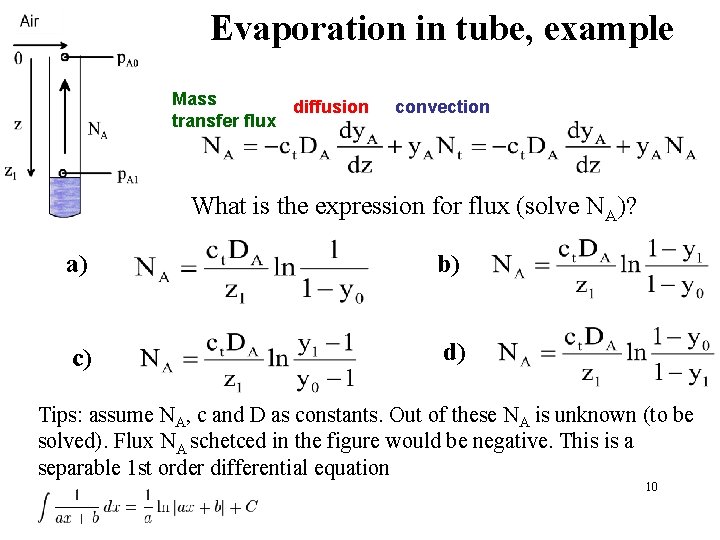
Evaporation in tube, example Mass diffusion transfer flux convection What is the expression for flux (solve NA)? a) b) c) d) Tips: assume NA, c and D as constants. Out of these NA is unknown (to be solved). Flux NA schetced in the figure would be negative. This is a separable 1 st order differential equation 10

Evaporation in tube, example 11

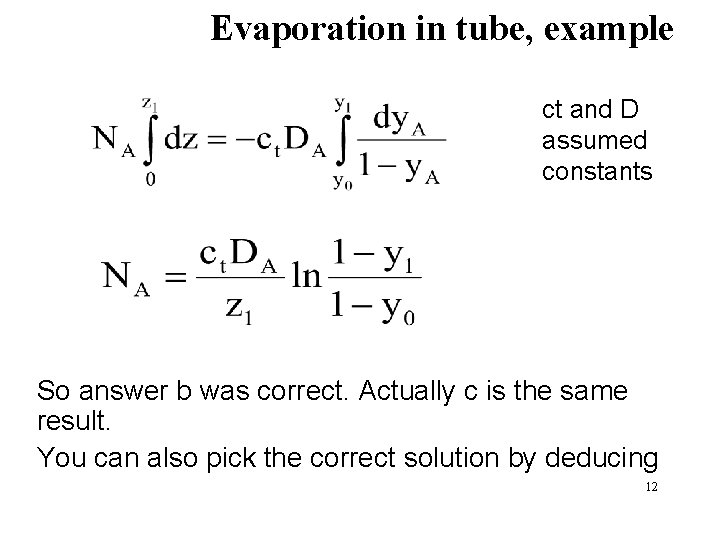
Evaporation in tube, example ct and D assumed constants So answer b was correct. Actually c is the same result. You can also pick the correct solution by deducing 12

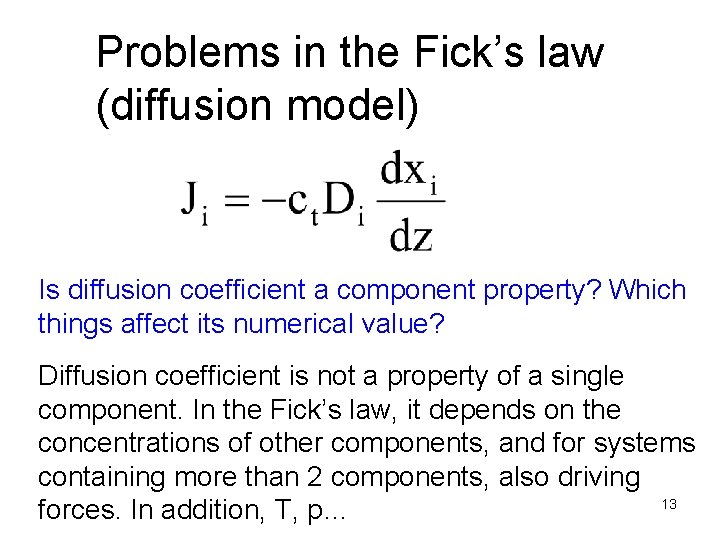
Problems in the Fick’s law (diffusion model) Is diffusion coefficient a component property? Which things affect its numerical value? Diffusion coefficient is not a property of a single component. In the Fick’s law, it depends on the concentrations of other components, and for systems containing more than 2 components, also driving 13 forces. In addition, T, p…

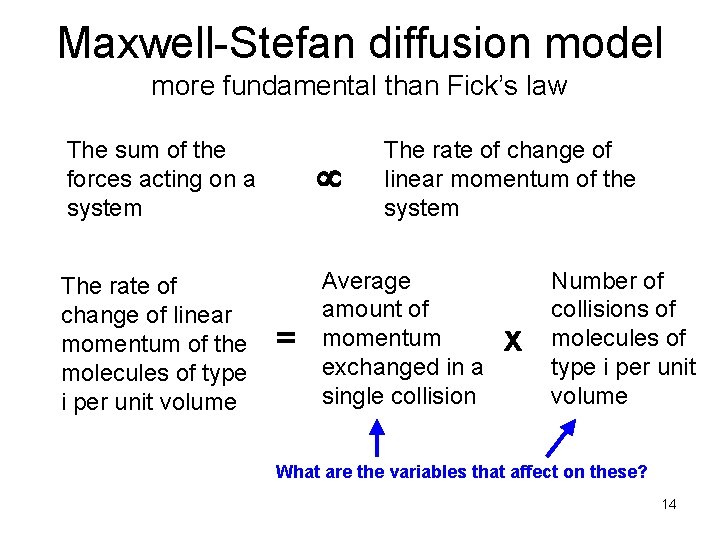
Maxwell-Stefan diffusion model more fundamental than Fick’s law The sum of the forces acting on a system The rate of change of linear momentum of the molecules of type i per unit volume Average amount of momentum exchanged in a single collision = The rate of change of linear momentum of the system x Number of collisions of molecules of type i per unit volume What are the variables that affect on these? 14

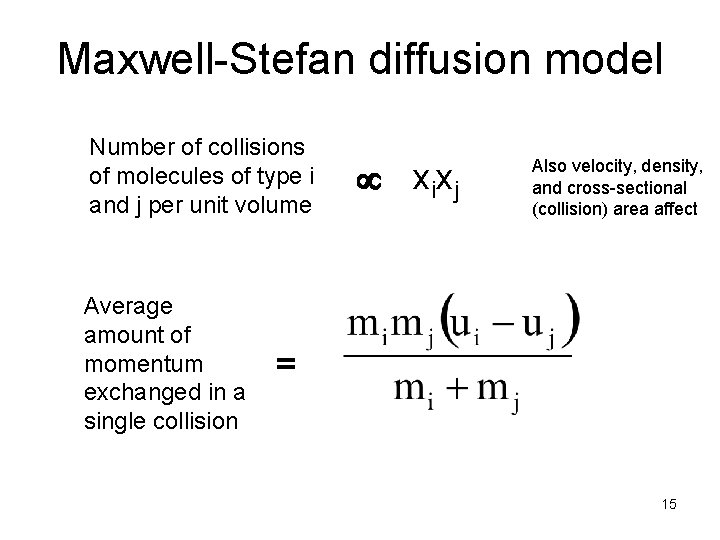
Maxwell-Stefan diffusion model Number of collisions of molecules of type i and j per unit volume Average amount of momentum exchanged in a single collision xixj Also velocity, density, and cross-sectional (collision) area affect = 15

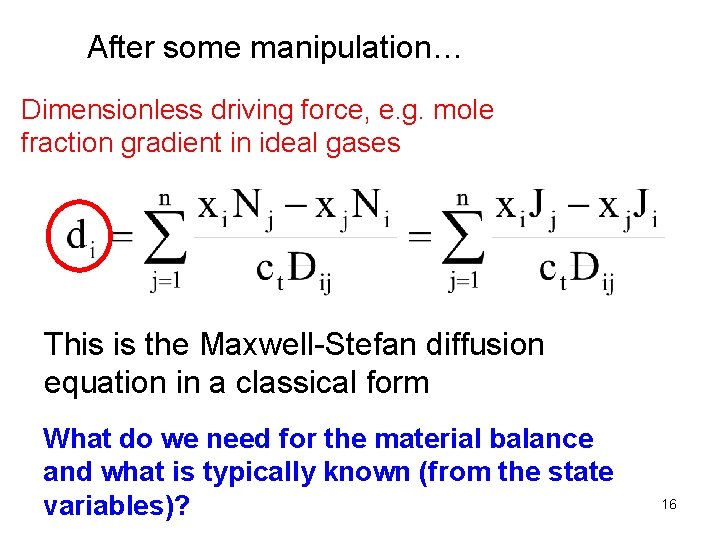
After some manipulation… Dimensionless driving force, e. g. mole fraction gradient in ideal gases This is the Maxwell-Stefan diffusion equation in a classical form What do we need for the material balance and what is typically known (from the state variables)? 16

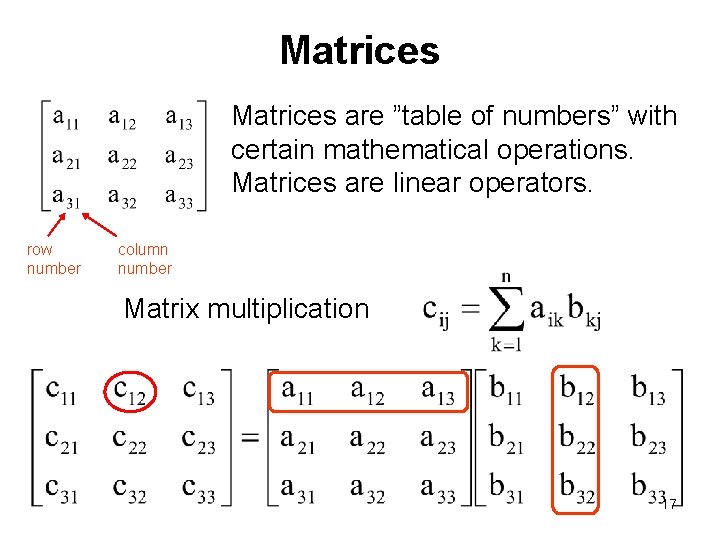
Matrices are ”table of numbers” with certain mathematical operations. Matrices are linear operators. row number column number Matrix multiplication 17

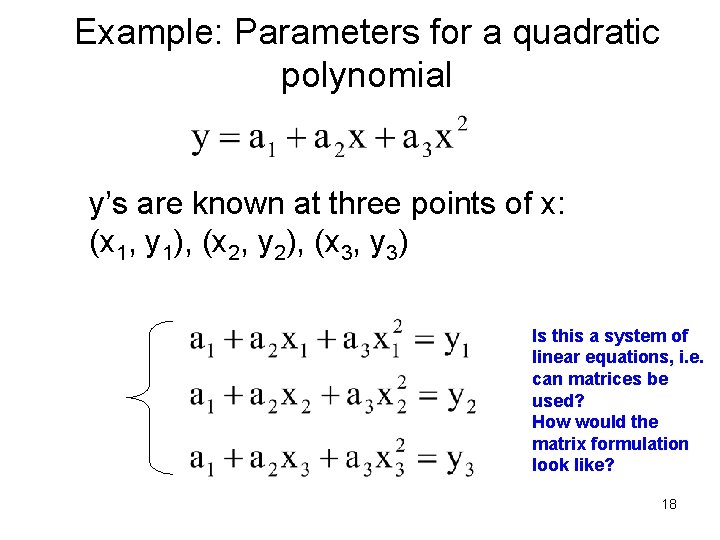
Example: Parameters for a quadratic polynomial y’s are known at three points of x: (x 1, y 1), (x 2, y 2), (x 3, y 3) Is this a system of linear equations, i. e. can matrices be used? How would the matrix formulation look like? 18

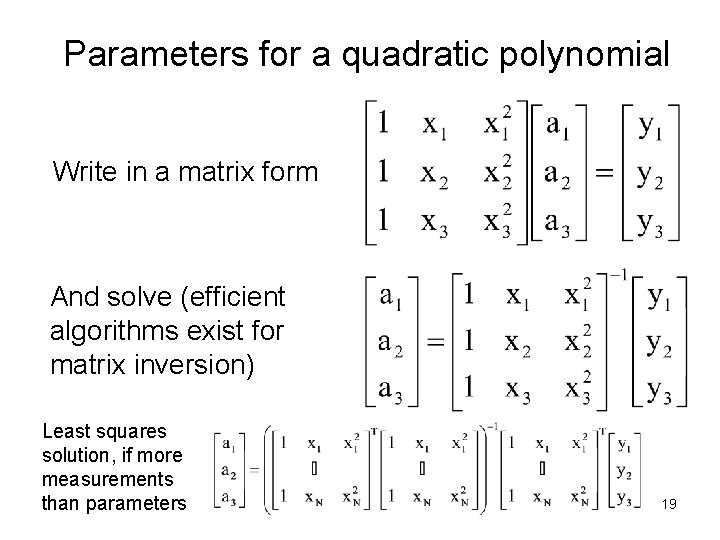
Parameters for a quadratic polynomial Write in a matrix form And solve (efficient algorithms exist for matrix inversion) Least squares solution, if more measurements than parameters 19

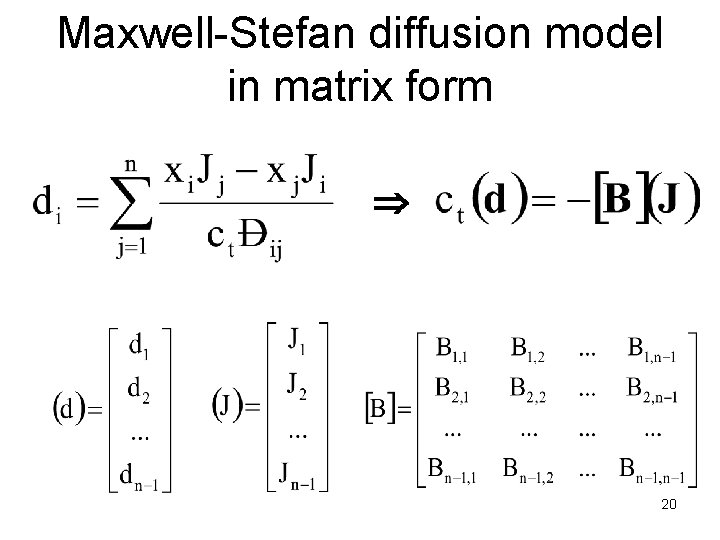
Maxwell-Stefan diffusion model in matrix form 20

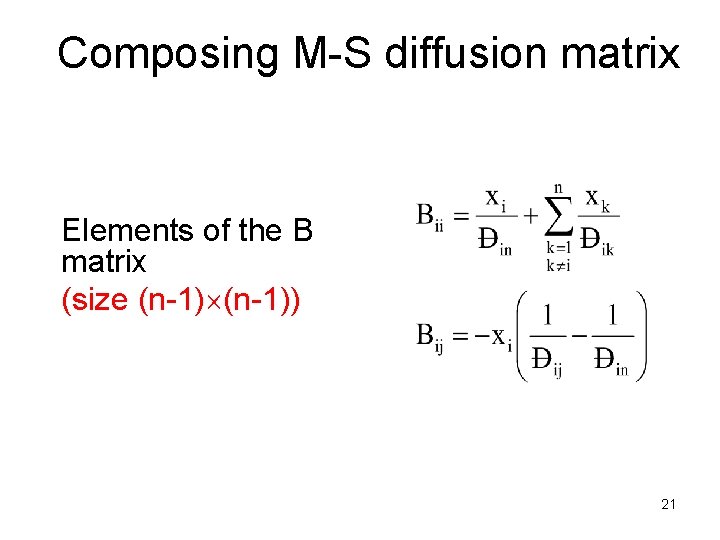
Composing M-S diffusion matrix Elements of the B matrix (size (n-1)) 21

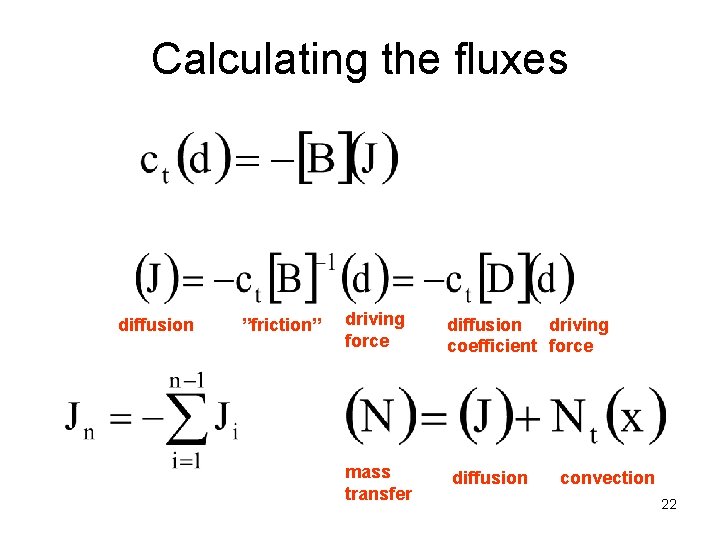
Calculating the fluxes diffusion ”friction” driving force mass transfer diffusion driving coefficient force diffusion convection 22

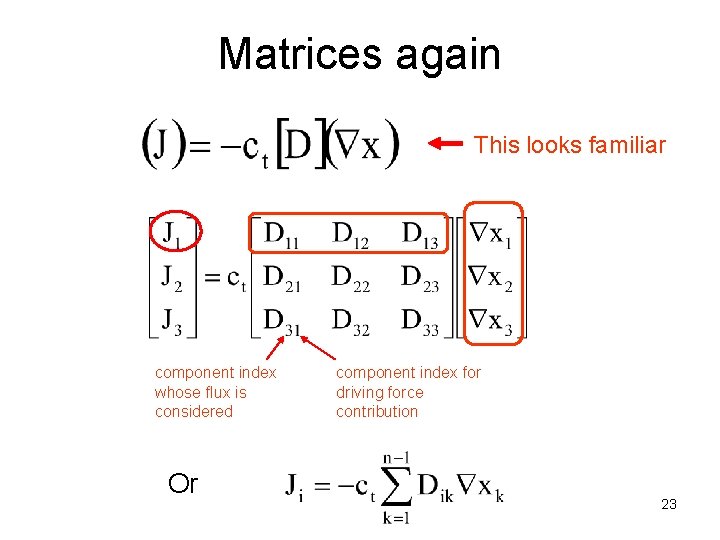
Matrices again This looks familiar component index whose flux is considered Or component index for driving force contribution 23

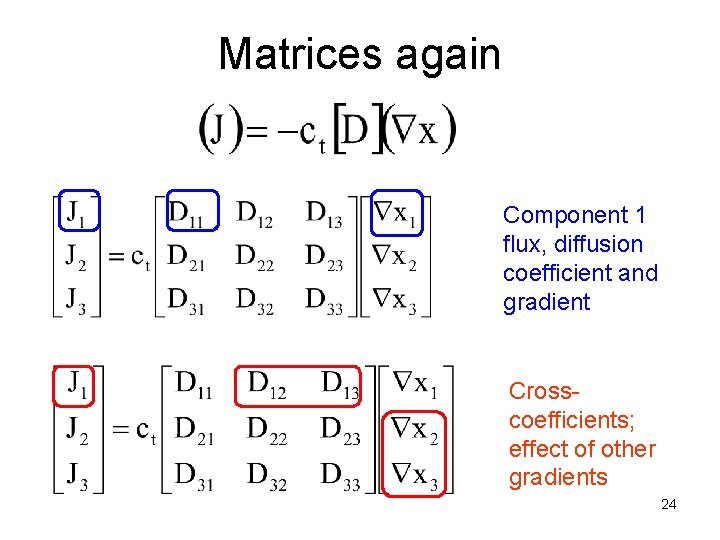
Matrices again Component 1 flux, diffusion coefficient and gradient Crosscoefficients; effect of other gradients 24

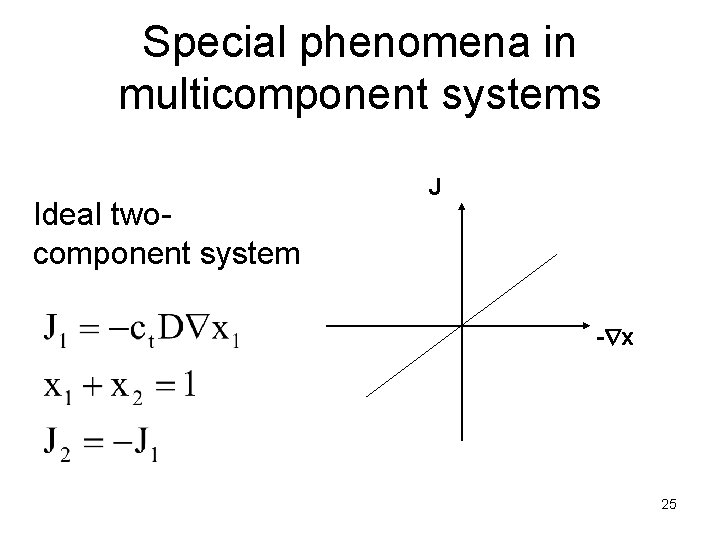
Special phenomena in multicomponent systems Ideal twocomponent system J - x 25

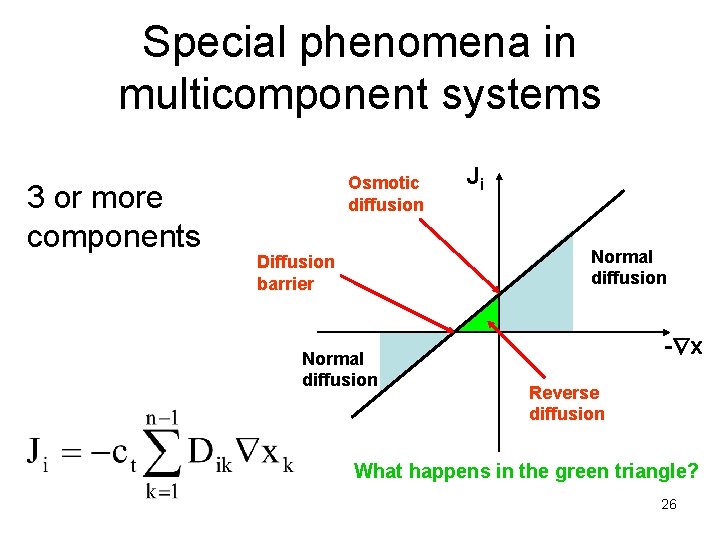
Special phenomena in multicomponent systems 3 or more components Osmotic diffusion Ji Normal diffusion Diffusion barrier Normal diffusion - x Reverse diffusion What happens in the green triangle? 26

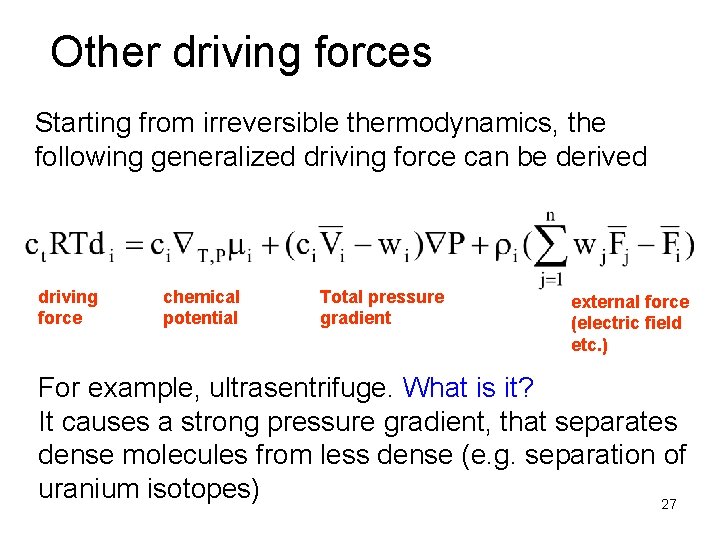
Other driving forces Starting from irreversible thermodynamics, the following generalized driving force can be derived driving force chemical potential Total pressure gradient external force (electric field etc. ) For example, ultrasentrifuge. What is it? It causes a strong pressure gradient, that separates dense molecules from less dense (e. g. separation of uranium isotopes) 27

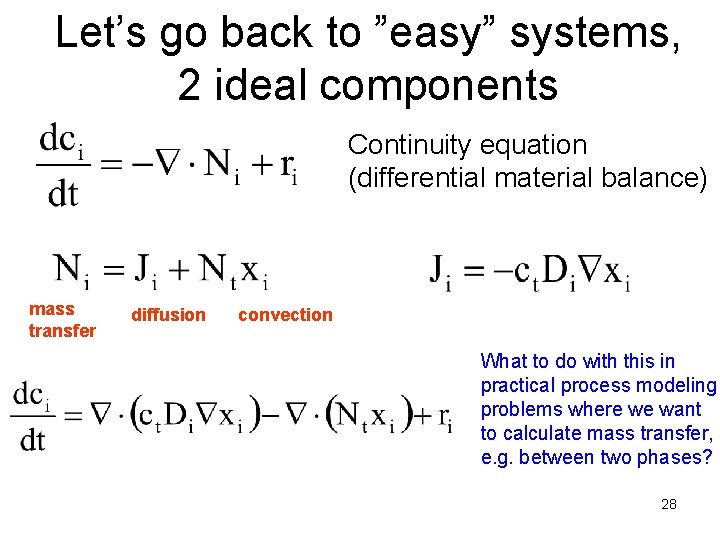
Let’s go back to ”easy” systems, 2 ideal components Continuity equation (differential material balance) mass transfer diffusion convection What to do with this in practical process modeling problems where we want to calculate mass transfer, e. g. between two phases? 28

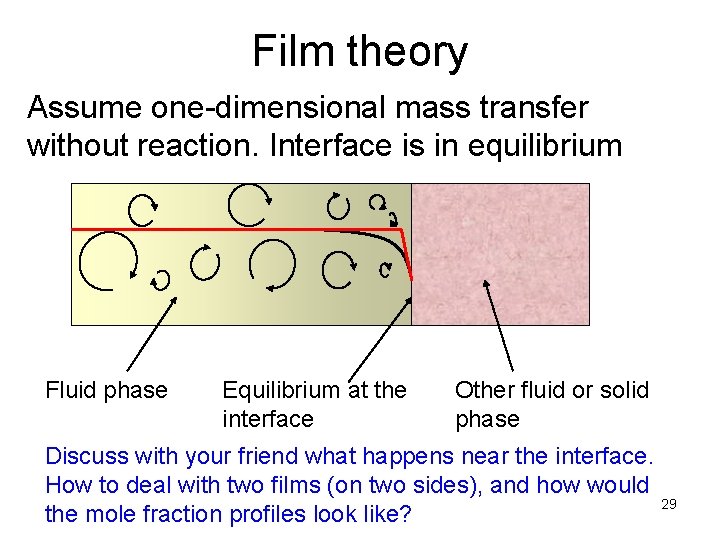
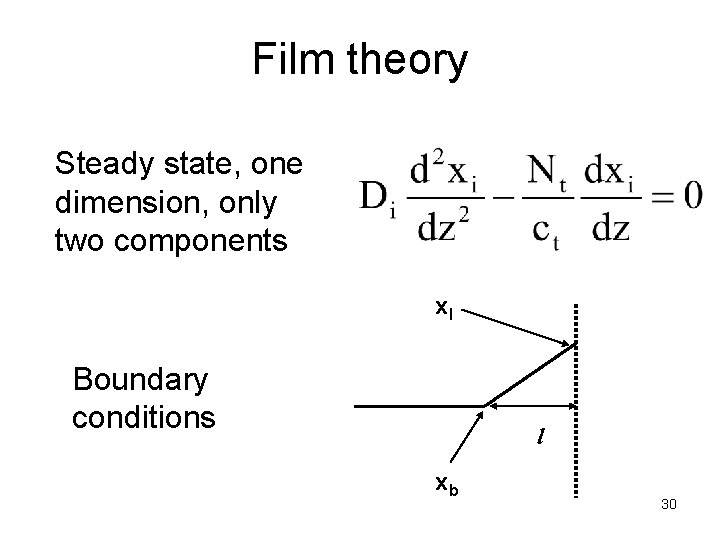
Film theory Assume one-dimensional mass transfer without reaction. Interface is in equilibrium Fluid phase Equilibrium at the interface Other fluid or solid phase Discuss with your friend what happens near the interface. How to deal with two films (on two sides), and how would 29 the mole fraction profiles look like?

Film theory Steady state, one dimension, only two components x. I Boundary conditions l xb 30

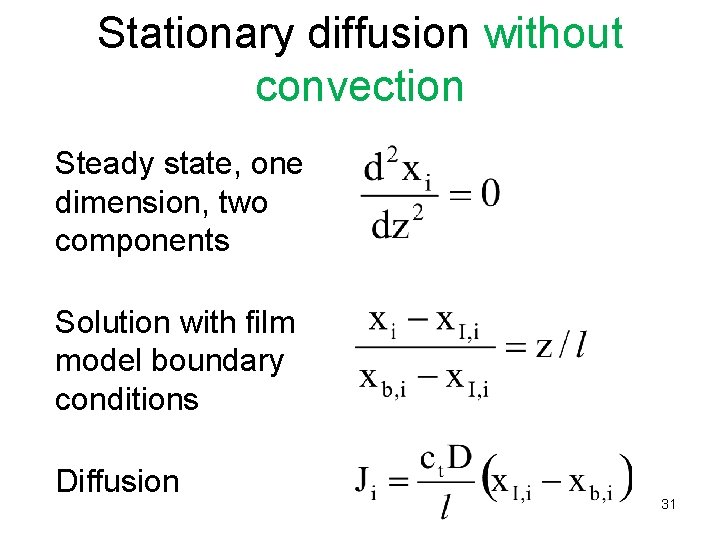
Stationary diffusion without convection Steady state, one dimension, two components Solution with film model boundary conditions Diffusion 31

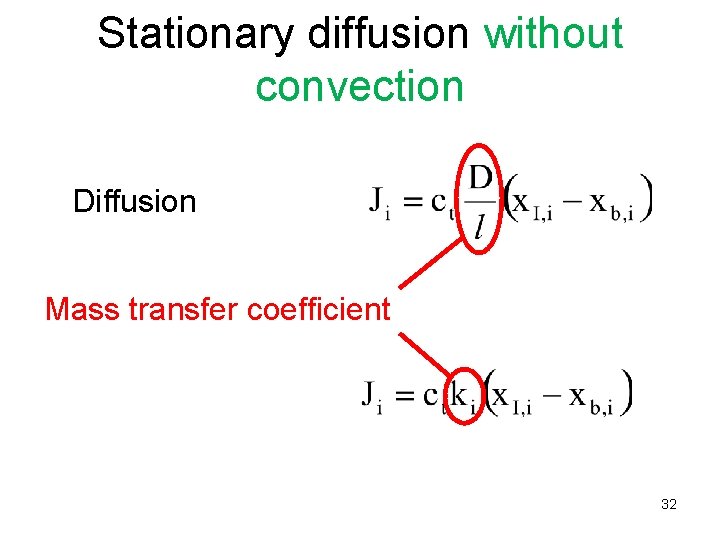
Stationary diffusion without convection Diffusion Mass transfer coefficient 32

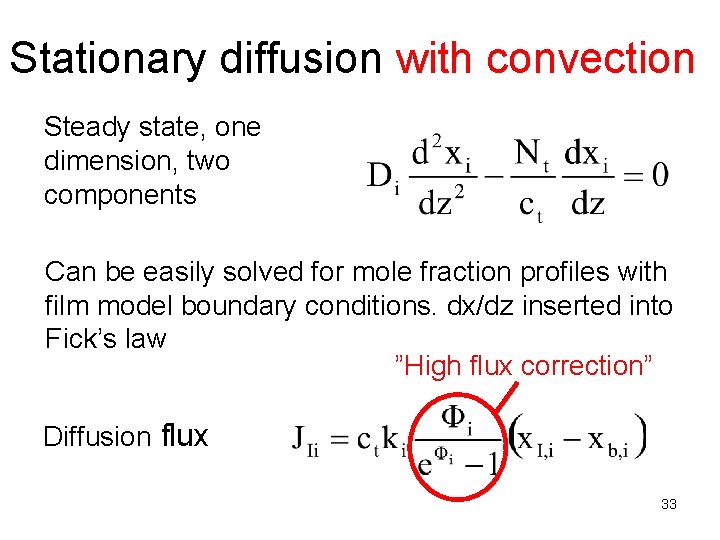
Stationary diffusion with convection Steady state, one dimension, two components Can be easily solved for mole fraction profiles with film model boundary conditions. dx/dz inserted into Fick’s law ”High flux correction” Diffusion flux 33

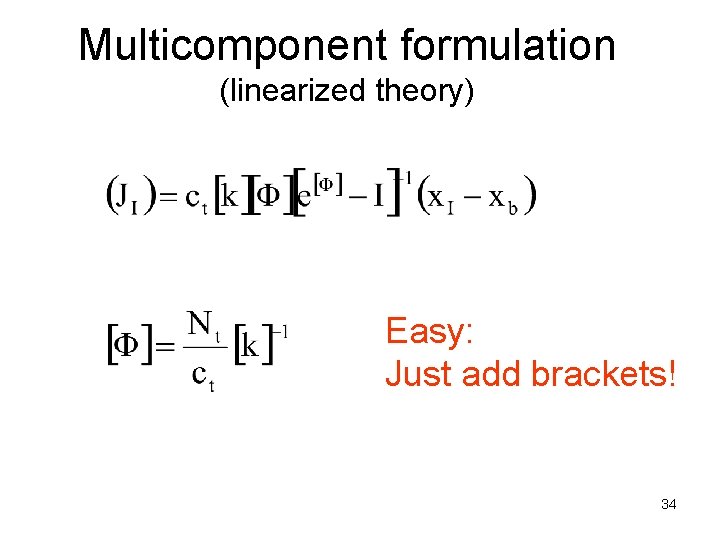
Multicomponent formulation (linearized theory) Easy: Just add brackets! 34

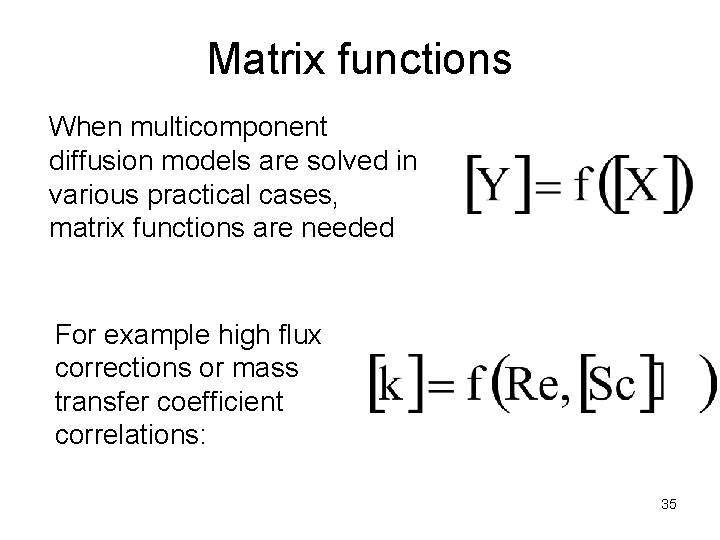
Matrix functions When multicomponent diffusion models are solved in various practical cases, matrix functions are needed For example high flux corrections or mass transfer coefficient correlations: 35

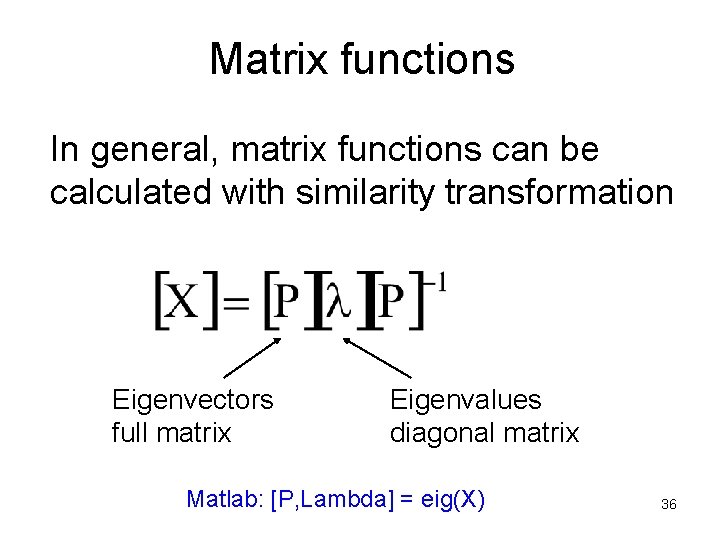
Matrix functions In general, matrix functions can be calculated with similarity transformation Eigenvectors full matrix Eigenvalues diagonal matrix Matlab: [P, Lambda] = eig(X) 36

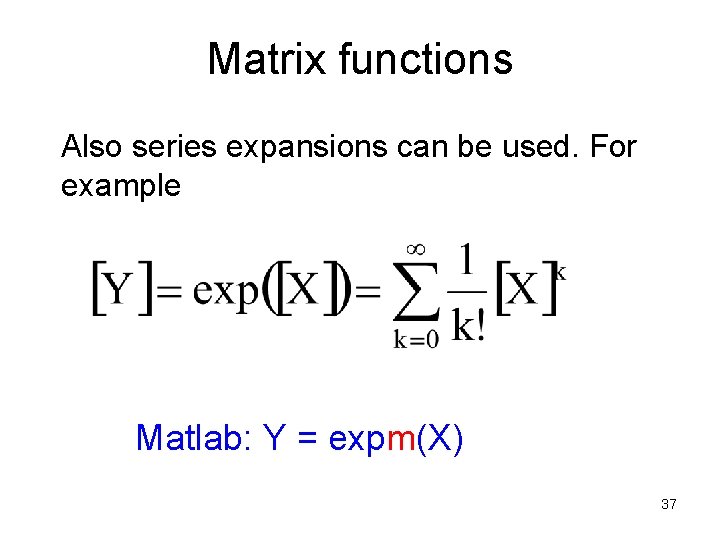
Matrix functions Also series expansions can be used. For example Matlab: Y = expm(X) 37

Solution of a two-film model with rigorous Maxwell-Stefan model Given: • bulk compositions • thermodynamic properties (equilibrium model, diffusion coefficients, etc. ) Unknown: mole fractions at the interface, total flux Nt 38

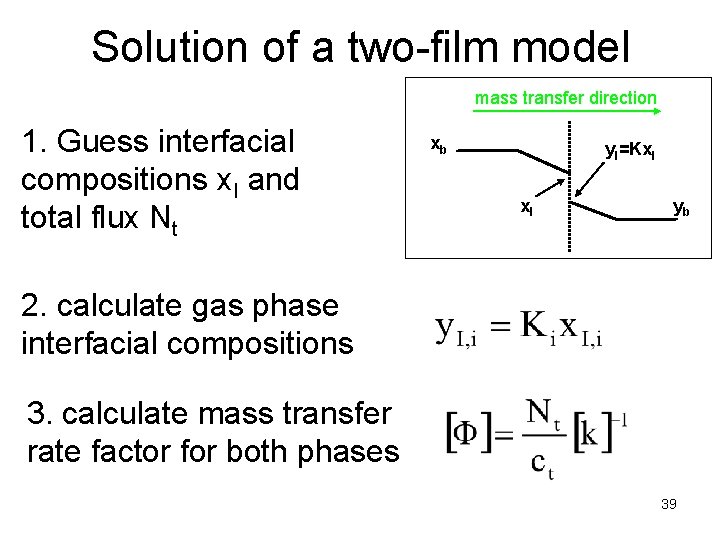
Solution of a two-film model mass transfer direction 1. Guess interfacial compositions x. I and total flux Nt xb y. I=Kx. I yb 2. calculate gas phase interfacial compositions 3. calculate mass transfer rate factor for both phases 39

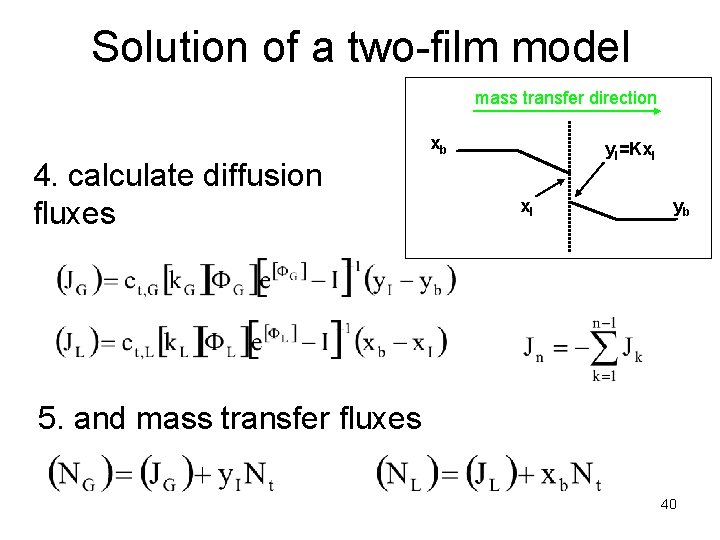
Solution of a two-film model mass transfer direction xb 4. calculate diffusion fluxes y. I=Kx. I yb 5. and mass transfer fluxes 40

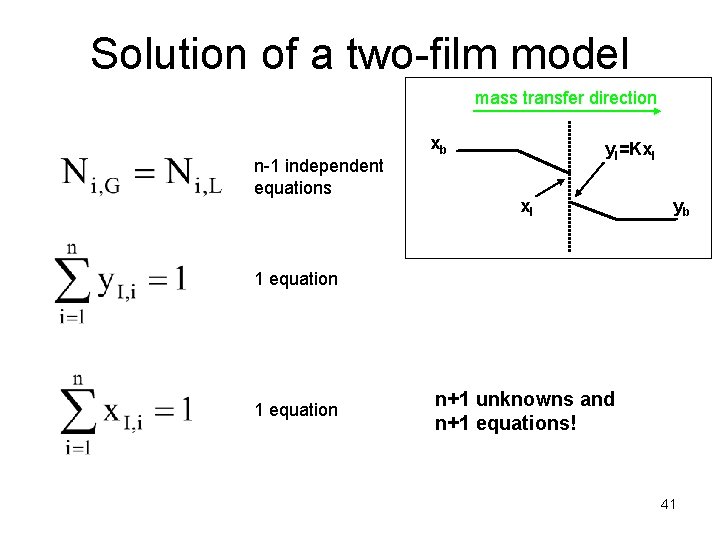
Solution of a two-film model mass transfer direction n-1 independent equations xb y. I=Kx. I yb 1 equation n+1 unknowns and n+1 equations! 41

Some common assumptions • Linearized mass transfer theory was already used (constant D and ct) • K values and [D] are calculated with bulk compositions • Fluid nonidealities neglected in driving force • Mass transfer coefficient matrix from binary coefficients, or by matrix approximations 42

Summary • Mass transfer is a process of approaching chemical equilibrium • A simple, ”engineering”, interpretation to mass transfer coefficient is the speed of approach to equilibrium • Maxwell-Stefan equations are based on conservation of momentum 43

Summary • In multicomponent systems, components can diffuse against their gradients due to interactions with other components • Generally, solution of multicomponent mass transfer models requires matrix function calculations • Film theory provides boundary conditions for practical mass transfer flux calculations 44