CHEM UNIT 3 PART 4 Lesson Periodic Table

CHEM – UNIT 3: PART 4 Lesson: Periodic Table

Learning Target • SWBAT explain an element’s properties based on its location on the periodic table. • Importance?

What is the periodic table? • “Periodic” means “repeated in a pattern” • Late 1800 s, Dmitri Mendeleev searched for a way to organize elements • 1913, Henry Moseley arranged elements based on atomic numbers

What is the periodic table? • Elements are arranged by increasing atomic number • Vertical columns (groups) • Elements in the same group have similar properties because they have the same number of valence electrons • Horizontal rows (periods) • End when an outer energy level is filled
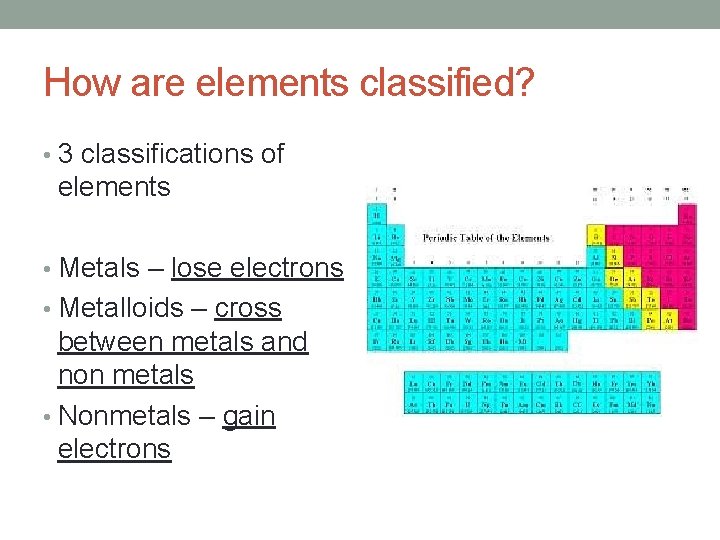
How are elements classified? • 3 classifications of elements • Metals – lose electrons • Metalloids – cross between metals and non metals • Nonmetals – gain electrons

Metals • Lose electrons • Found on the left side of the periodic table • Conduct electricity • Solid at room temperature (except for Mercury (Hg)) • Luster – reflect light • Malleable – can be hammered or rolled • Ductile – can be drawn into wires

Metalloids • Metallic and nonmetallic properties • Stair steps on the table • Aluminum is included • “Semiconductors” • Conduct electricity but not as well as metals
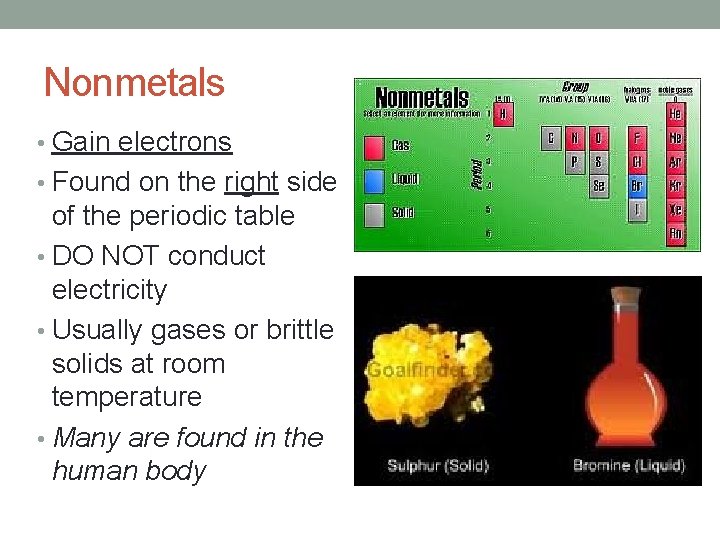
Nonmetals • Gain electrons • Found on the right side of the periodic table • DO NOT conduct electricity • Usually gases or brittle solids at room temperature • Many are found in the human body

Periodic Table Gallery Walk Group Name 1 1 2 3 – 12 17 18 Hydrogen Characteristics
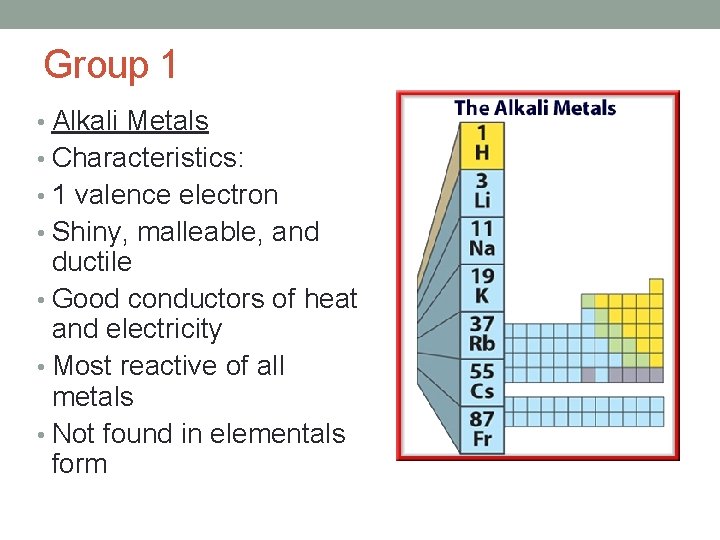
Group 1 • Alkali Metals • Characteristics: • 1 valence electron • Shiny, malleable, and ductile • Good conductors of heat and electricity • Most reactive of all metals • Not found in elementals form
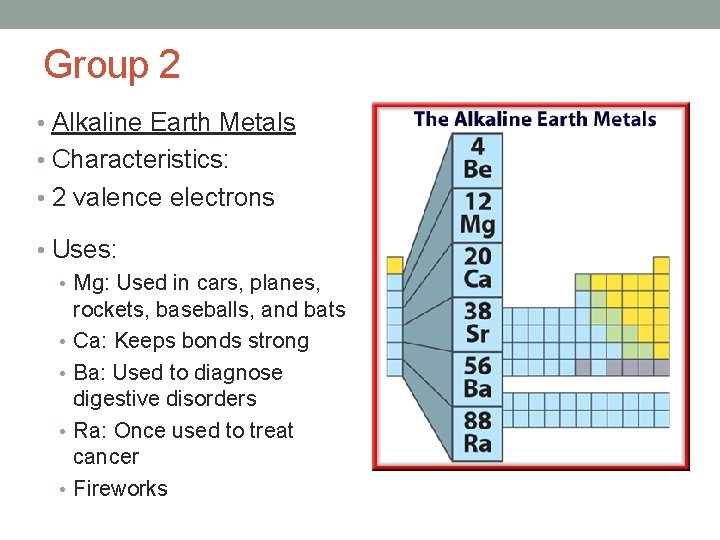
Group 2 • Alkaline Earth Metals • Characteristics: • 2 valence electrons • Uses: • Mg: Used in cars, planes, rockets, baseballs, and bats • Ca: Keeps bonds strong • Ba: Used to diagnose digestive disorders • Ra: Once used to treat cancer • Fireworks
Groups 3 – 12 • Transition Metals • Characteristics: • Form colored compounds • Occur in nature as uncombined elements • Conduct electricity • Many can form multiple ions
Group 1: Hydrogen • Characteristics: • 1 proton, 1 electron • Very reactive • Makes up 90% of all atoms in the universe
Group 17 • Halogens • Characteristics: • 7 valence electrons • Very reactive in elemental form • Fluorine is the most chemically active element • Uses: Lights, semiconductors, glass etching
Group 18 • Noble Gases • Characteristics: • 8 valence electrons • Stable • Do not form compounds • Exist as gases
- Slides: 15