CHEM SOL REVIEW DAY 4 SOLS 1 5










- Slides: 24

CHEM SOL REVIEW DAY 4 SOLS 1 & 5

SCIENTIFIC INVESTIGATIONS A __________ is an explanation that might be true and can be tested. Hypothesis The factor that is changed in the experimental group is the _____ variable. It is plotted on the x-axis. Independent

SCIENTIFIC INVESTIGATIONS What you measure in an experiment is called the ________ variable. It is plotted on the y-axis. Dependent Name 3 good safety rules for a Chemistry lab.

UNIT 1 – SCIENTIFIC MEASUREMENT Write the following numbers in scientific notation. 52, 300 5. 23 x 104 0. 035 3. 5 x 10 -2

UNIT 1 – SCIENTIFIC MEASUREMENT The measure of how close a measurement is to what it should be is called the ____ Accuracy The repeatability of a measurement is called ____ Precision

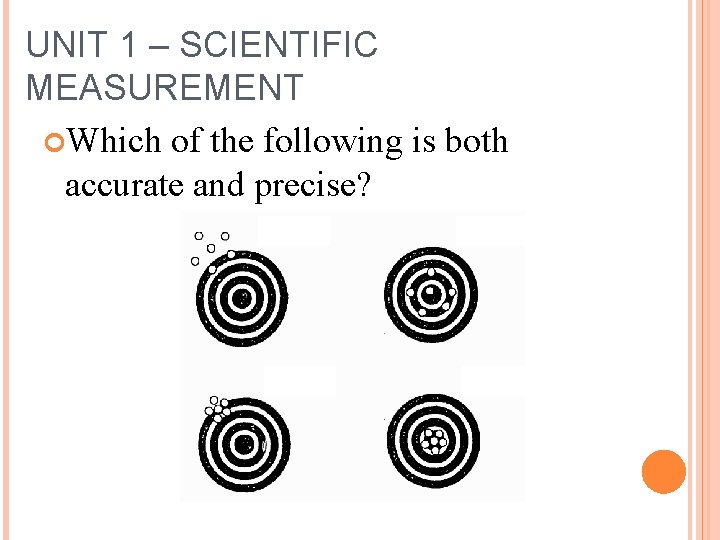
UNIT 1 – SCIENTIFIC MEASUREMENT Which of the following is both accurate and precise?

UNIT 1 – SCIENTIFIC MEASUREMENT I conduct an experiment and measure the mass of a substance as 7. 31 g. The accepted value is 7. 5 g. What is the percent error? (7. 5 -7. 31) / 7. 5 x 100 2. 5%

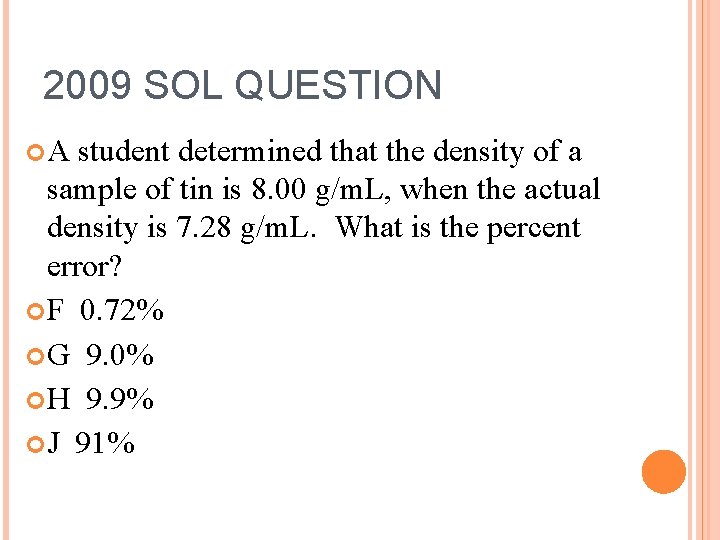
2009 SOL QUESTION A student determined that the density of a sample of tin is 8. 00 g/m. L, when the actual density is 7. 28 g/m. L. What is the percent error? F 0. 72% G 9. 0% H 9. 9% J 91%

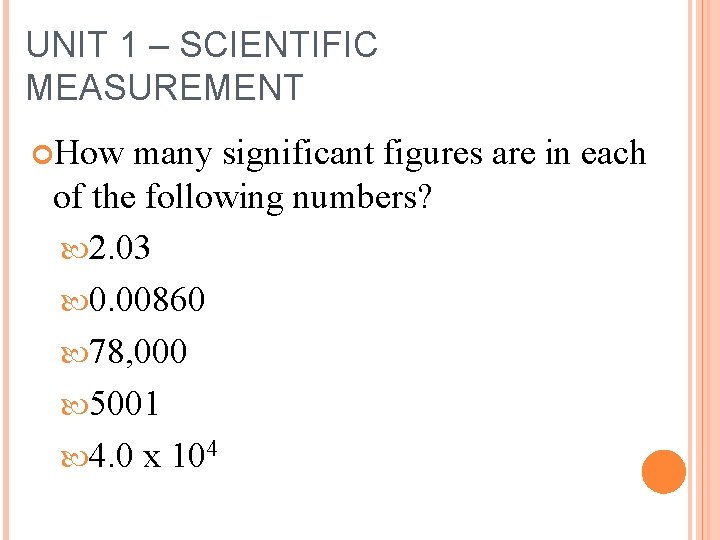
UNIT 1 – SCIENTIFIC MEASUREMENT How many significant figures are in each of the following numbers? 2. 03 0. 00860 78, 000 5001 4. 0 x 104

2009 SOL QUESTION A compound has a mass of 2. 6632 x 102 g/mol. The number of sig figs in this mass is – F 2 G 4 H 5 J 7

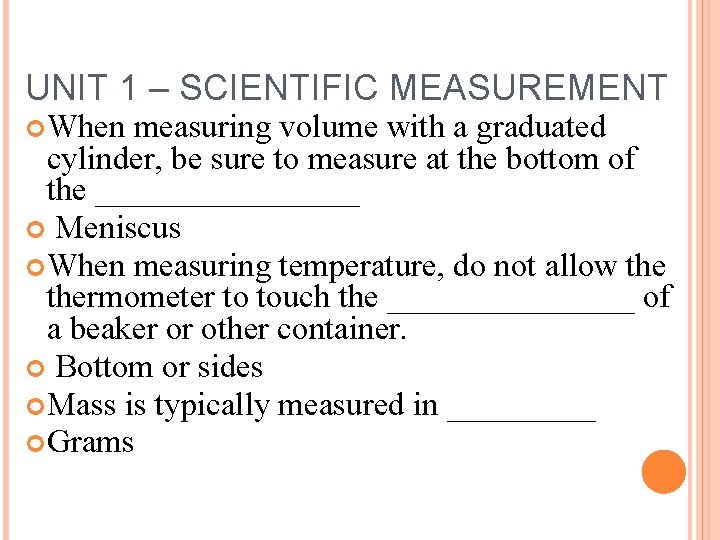
UNIT 1 – SCIENTIFIC MEASUREMENT When measuring volume with a graduated cylinder, be sure to measure at the bottom of the ________ Meniscus When measuring temperature, do not allow thermometer to touch the ________ of a beaker or other container. Bottom or sides Mass is typically measured in _____ Grams

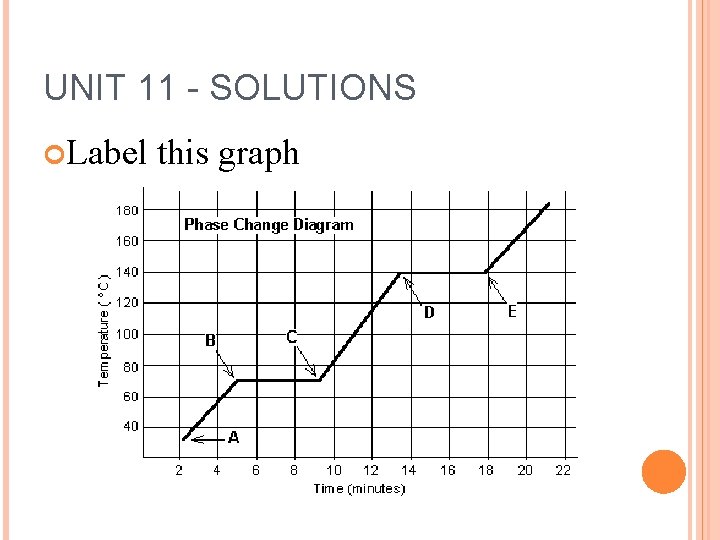
UNIT 11 - SOLUTIONS Label this graph

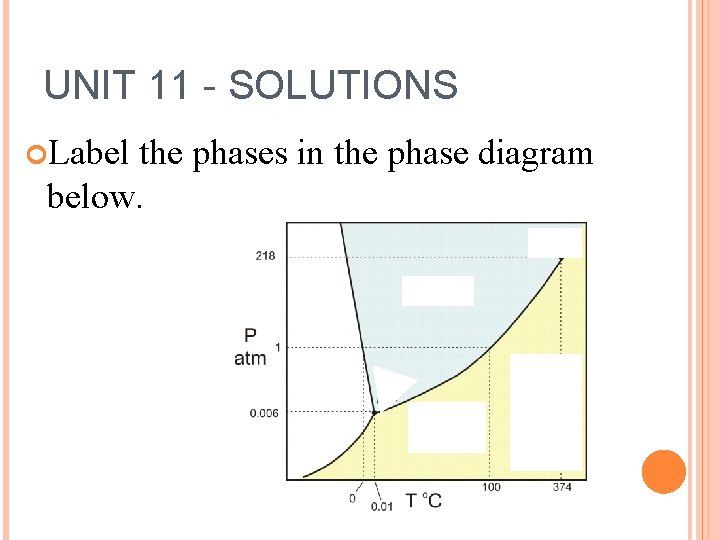
UNIT 11 - SOLUTIONS Label the phases in the phase diagram below.

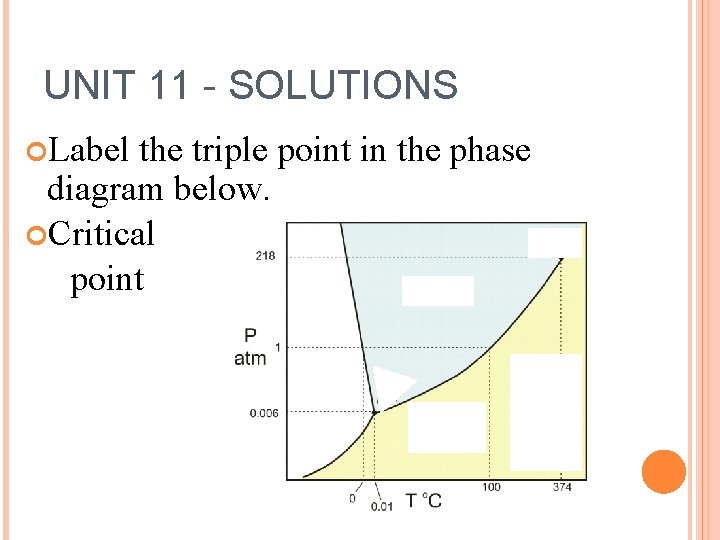
UNIT 11 - SOLUTIONS Label the triple point in the phase diagram below. Critical point

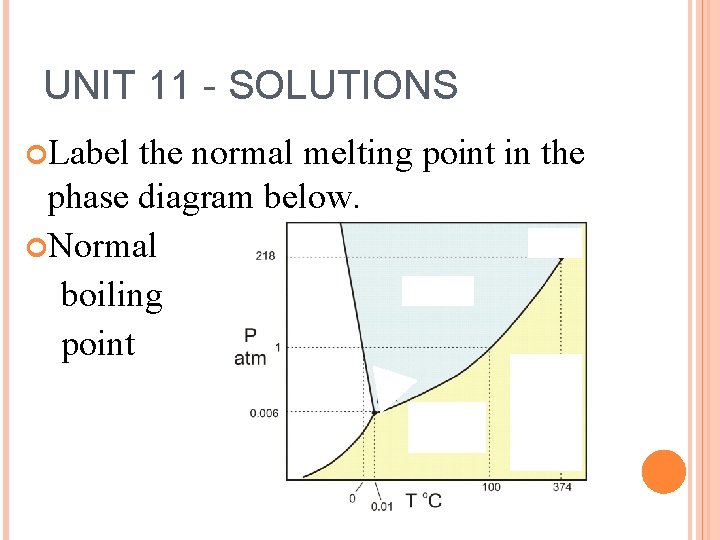
UNIT 11 - SOLUTIONS Label the normal melting point in the phase diagram below. Normal boiling point

2009 SOL QUESTION When 1 g of Na. Cl is placed in 100 g of water, a solution results. Once the solution is prepared, water is now considered what part of the solution? F Solute G Solvent

UNIT 13 – EVERYTHING ELSE Vaporization is an _____ process. Endothermic Freezing is an ______process. Exothermic Heat (q) is the ____ of energy between objects at different temperatures. Transfer

UNIT 13 – EVERYTHING ELSE Heat is an ____ property --- it depends on the amount of the substance present. Extensive The total energy content of a sample is called its _____. Enthalpy

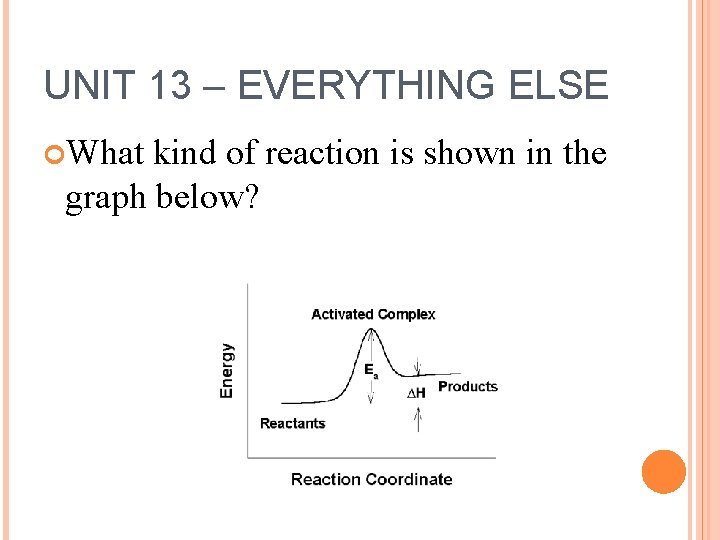
UNIT 13 – EVERYTHING ELSE What kind of reaction is shown in the graph below?

UNIT 13 – EVERYTHING ELSE The measure of disorder or randomness in a system is called the ___________. Entropy A catalyst is a substance that speeds up the reaction by ___ the activation energy. Lowering Increasing the temperature, concentration, pressure, or surface area of a substance will ______ the reaction rate. Increase

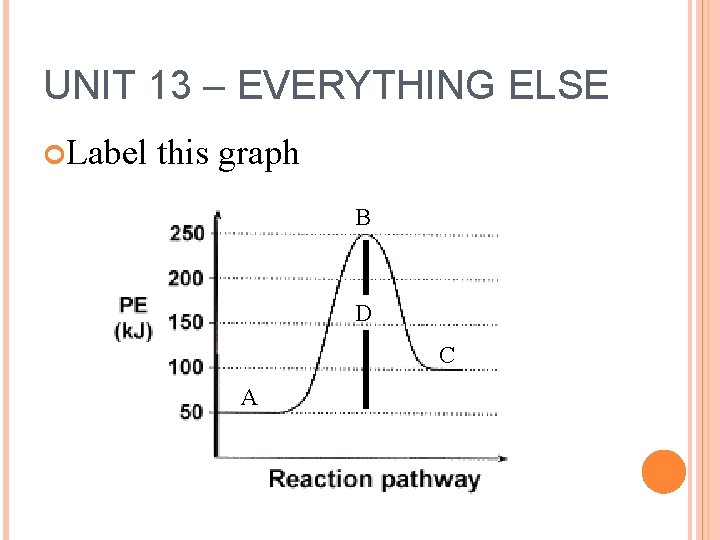
UNIT 13 – EVERYTHING ELSE Label this graph B D C A

UNIT 13 – EVERYTHING ELSE When the forward and reverse reactions happen at the same rate, it is called ___ Chemical equilibrium Give an example of a completion reaction. Le Chatelier’s principle states that when a system is at equilibrium is disturbed, the system adjusts in a way to _______ the change. Reduce

UNIT 13 – EVERYTHING ELSE Increasing the concentration of the reactants will make the ______ reaction faster than the reverse. Forward This is called a shift _____. Right An oxidation reaction is one in which electrons are _____. Lost

UNIT 13 – EVERYTHING ELSE Assign oxidation numbers to each element in each of the following. K+ 1+ O 2 0 Ca. H 2 Ca = 2+, H =1 NH 4 H = 1+, N = 5 -