Chem E 260 Transient Mass and Energy Balances
Chem. E 260 Transient Mass and Energy Balances Dr. William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 5: D & E CB 4: 5 April 20, 2005
Transient Processes • If ANY variable associated with the process changes over time, then it is a transient process. • Transient processes are very complex and a computer is often required to solve the equations that describe these processes. • We will only consider special transient processes in which the following assumptions are true. • Uniform Flow – The properties and flow rates of all inlet and outlet streams are constant or uniform over the cross-sectional area for flow and are also constant with respect to time. • Uniform State – The state of the mass within the system is uniform. – At all times, the properties of the outlet stream are exactly the same as the properties of the system at that time. – The properties of the system can change with time, but they are always the same as the properties of the outlet stream Baratuci Chem. E 260 April 20, 2005
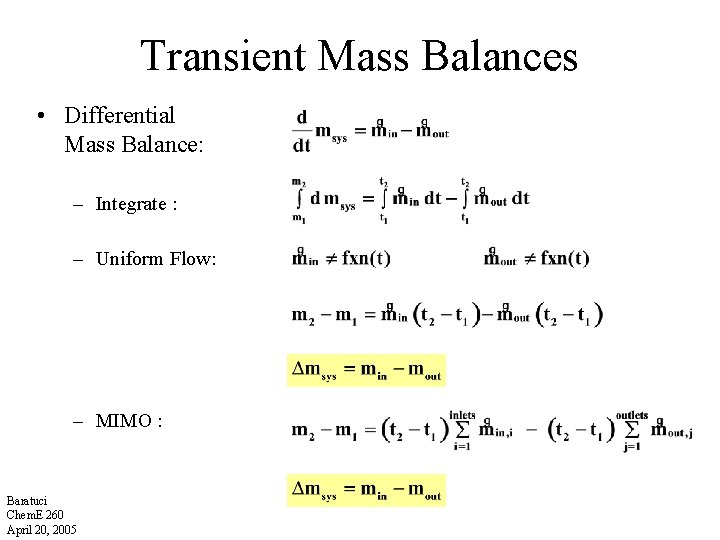
Transient Mass Balances • Differential Mass Balance: – Integrate : – Uniform Flow: – MIMO : Baratuci Chem. E 260 April 20, 2005
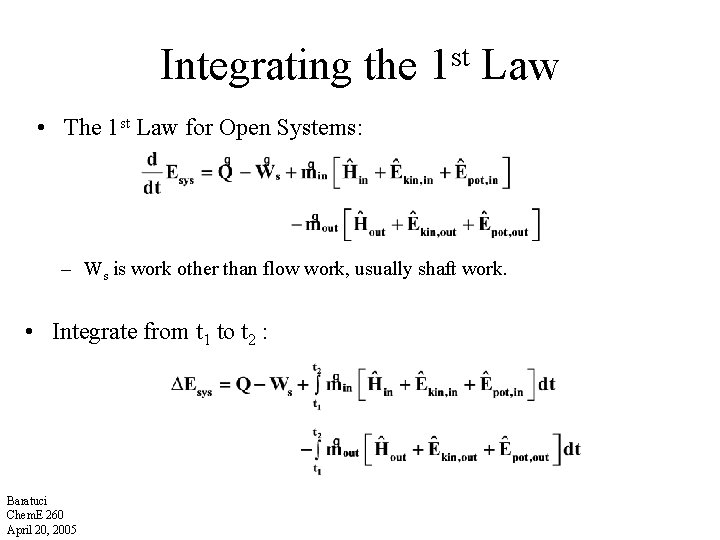
Integrating the 1 st Law • The 1 st Law for Open Systems: – Ws is work other than flow work, usually shaft work. • Integrate from t 1 to t 2 : Baratuci Chem. E 260 April 20, 2005
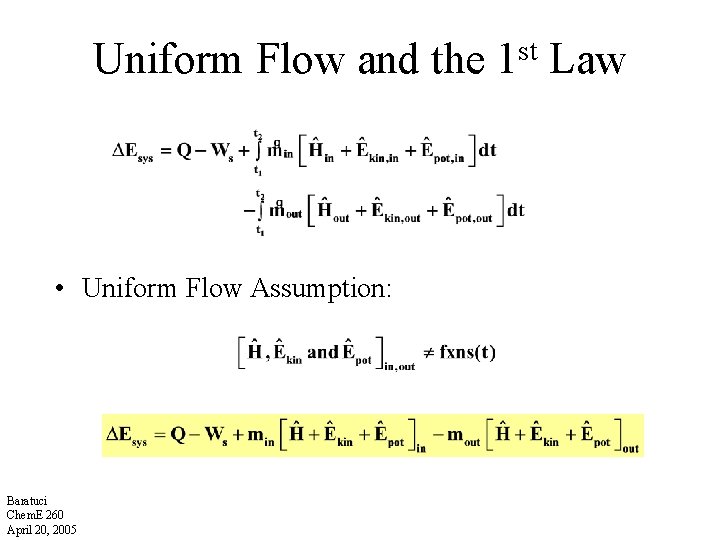
Uniform Flow and the • Uniform Flow Assumption: Baratuci Chem. E 260 April 20, 2005 st 1 Law
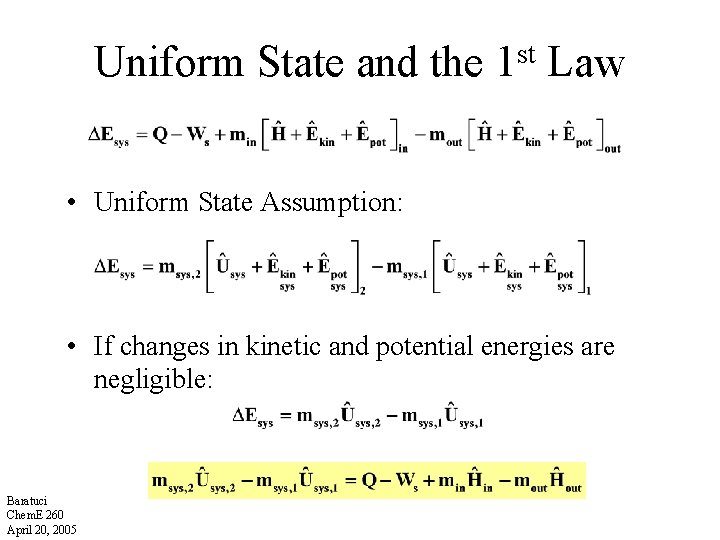
Uniform State and the st 1 Law • Uniform State Assumption: • If changes in kinetic and potential energies are negligible: Baratuci Chem. E 260 April 20, 2005
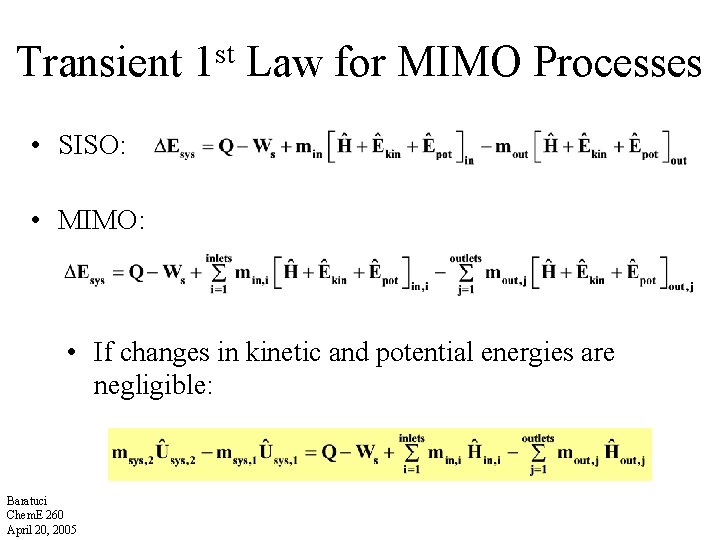
Transient st 1 Law for MIMO Processes • SISO: • MIMO: • If changes in kinetic and potential energies are negligible: Baratuci Chem. E 260 April 20, 2005
Next Class … • Problem Session • After that… • TCD 6: A & B and CB 5: 1 - 3 – Introduction to the 2 nd Law of Thermodynamics – Heat Engines & Thermal Reservoirs Baratuci Chem. E 260 April 20, 2005
Example Problem • Steam at a pressure of 1. 4 Mpa and 300 o. C is flowing through a pipe. Connected to this pipe through a valve is an evacuated tank. The valve is opened and the tank fills with steam until the pressure in the tank is also 1. 4 Mpa. Then, the valve is closed. The process is adiabatic and changes in kinetic and potential energies are negligible. Determin the final temperature of the steam in the tank. Baratuci Chem. E 260 April 20, 2005
- Slides: 9