Chem E 260 Internal Energy Enthalpy The NIST



- Slides: 12

Chem. E 260 Internal Energy, Enthalpy & The NIST Webbook Dr. William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 3: A & B CB 2: 9 – 11, Supplement April 5, 2005

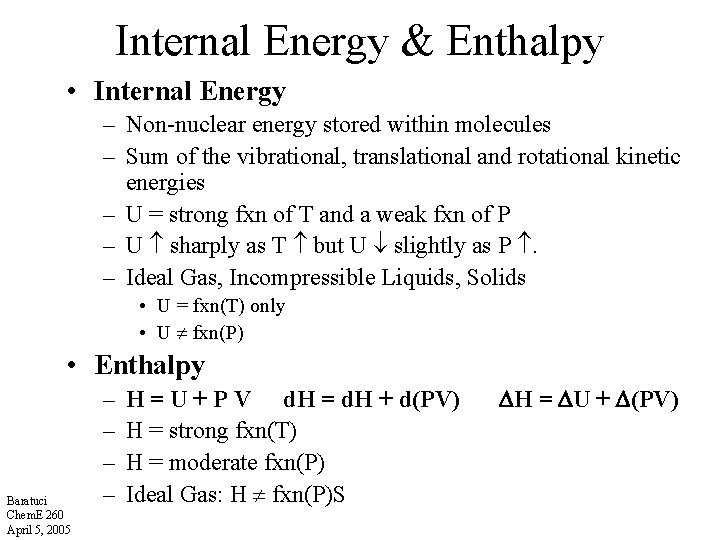
Internal Energy & Enthalpy • Internal Energy – Non-nuclear energy stored within molecules – Sum of the vibrational, translational and rotational kinetic energies – U = strong fxn of T and a weak fxn of P – U sharply as T but U slightly as P . – Ideal Gas, Incompressible Liquids, Solids • U = fxn(T) only • U fxn(P) • Enthalpy Baratuci Chem. E 260 April 5, 2005 – – H = U + P V d. H = d. H + d(PV) H = strong fxn(T) H = moderate fxn(P) Ideal Gas: H fxn(P)S H = U + (PV)

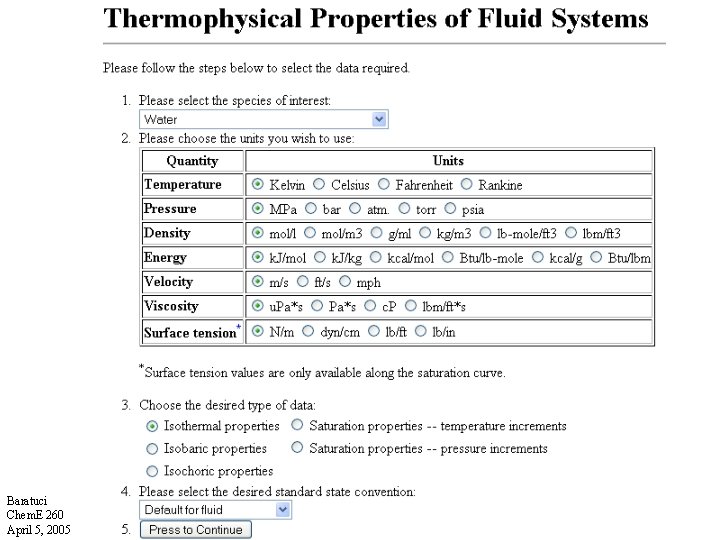
NIST Webbook Baratuci Chem. E 260 April 5, 2005

Reference State • We cannot determine an absolute U or H in the way we can determine an absolute T. • We must choose a reference state and assign = 0 or = 0 at that state. • Calculate all other values of and relative to the reference state. • You cannot use thermodynamic data from different sources that are based on different reference states without correcting for the difference in reference state !! Baratuci Chem. E 260 April 5, 2005

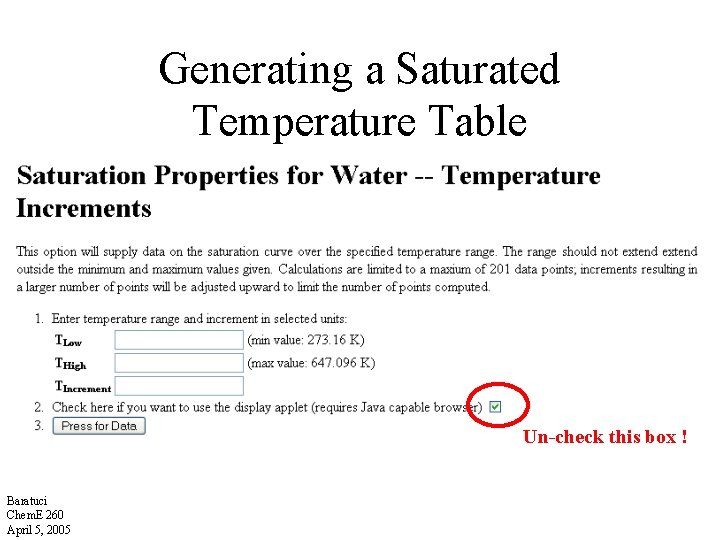
Generating a Saturated Temperature Table Un-check this box ! Baratuci Chem. E 260 April 5, 2005

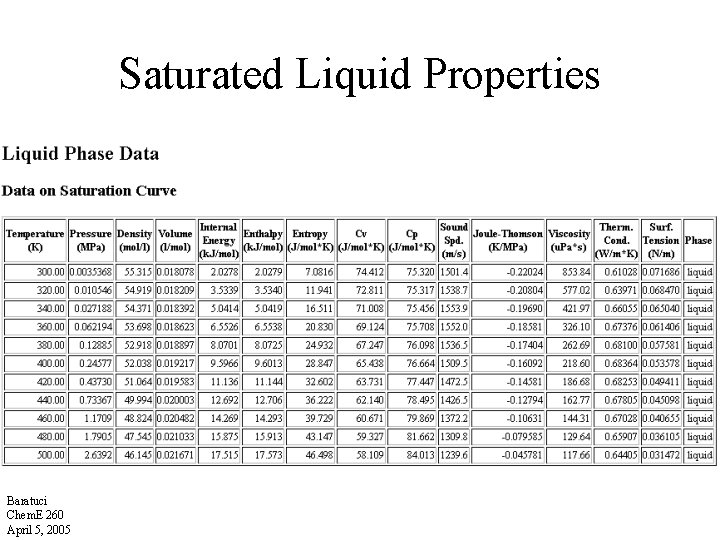
Saturated Liquid Properties Baratuci Chem. E 260 April 5, 2005

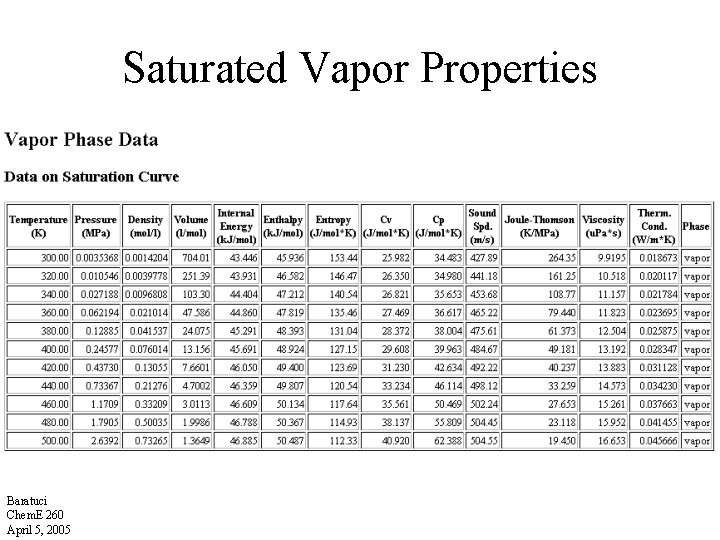
Saturated Vapor Properties Baratuci Chem. E 260 April 5, 2005

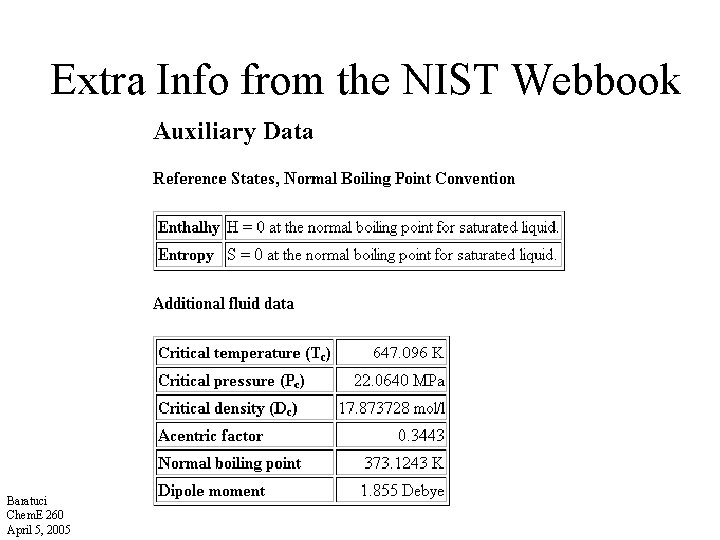
Extra Info from the NIST Webbook Baratuci Chem. E 260 April 5, 2005

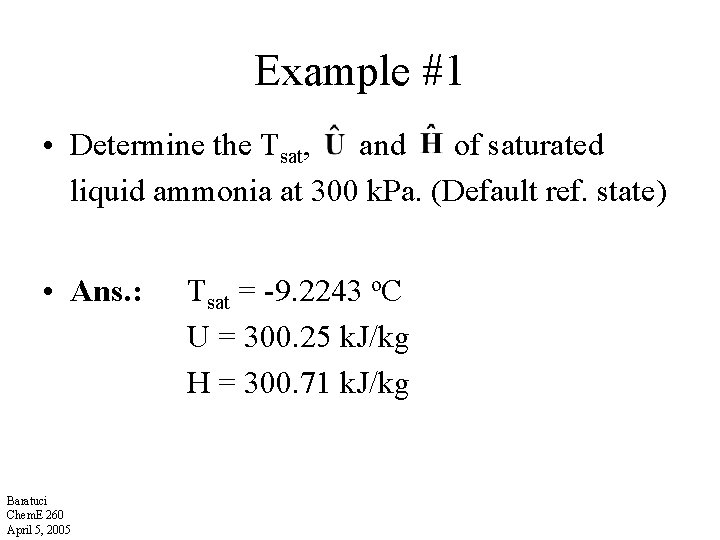
Example #1 • Determine the Tsat, and of saturated liquid ammonia at 300 k. Pa. (Default ref. state) • Ans. : Baratuci Chem. E 260 April 5, 2005 Tsat = -9. 2243 o. C U = 300. 25 k. J/kg H = 300. 71 k. J/kg

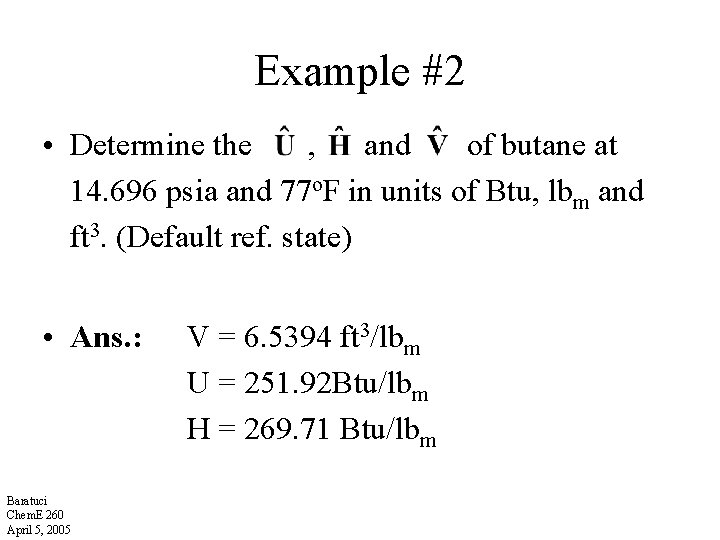
Example #2 • Determine the , and of butane at 14. 696 psia and 77 o. F in units of Btu, lbm and ft 3. (Default ref. state) • Ans. : Baratuci Chem. E 260 April 5, 2005 V = 6. 5394 ft 3/lbm U = 251. 92 Btu/lbm H = 269. 71 Btu/lbm

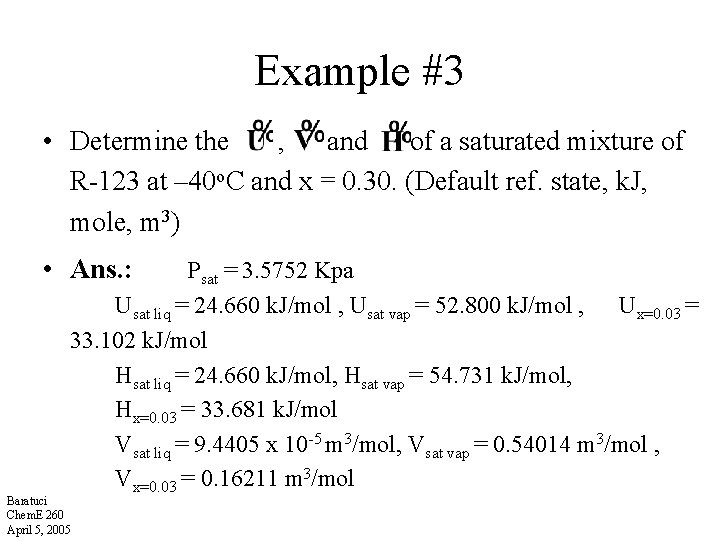
Example #3 • Determine the , and of a saturated mixture of R-123 at – 40 o. C and x = 0. 30. (Default ref. state, k. J, mole, m 3) • Ans. : Psat = 3. 5752 Kpa Usat liq = 24. 660 k. J/mol , Usat vap = 52. 800 k. J/mol , Ux=0. 03 = 33. 102 k. J/mol Hsat liq = 24. 660 k. J/mol, Hsat vap = 54. 731 k. J/mol, Hx=0. 03 = 33. 681 k. J/mol Vsat liq = 9. 4405 x 10 -5 m 3/mol, Vsat vap = 0. 54014 m 3/mol , Vx=0. 03 = 0. 16211 m 3/mol Baratuci Chem. E 260 April 5, 2005

Next Class • Heat Capacities – How much does the temperature of 1 mole or kg of a substance change when 1 J is added ? • Phase Changes – Latent heats of vaporization, fusion and sublimation • Hypothetical Process Paths – HPP’s make it easier to calculate how much a property changes during any real process. Baratuci Chem. E 260 April 5, 2005