Chem Catalyst These diagrams are called Lewis dot








- Slides: 14

Chem. Catalyst These diagrams are called Lewis dot symbols. 1. What is the relationship between the number of dots, the number of valence electrons, and the HONC 1234 rule? 2. Create a Lewis dot symbol for fluorine, F. How many bonds will fluorine make?

Draw the Lewis dot structure for the following elements (write e- config first): 4 valence e- Si 1 s 2 2 p 6 3 s 2 3 p 2 O 1 s 2 2 p 4 6 valence e- P 1 s 2 2 p 6 3 s 2 3 p 3 5 valence e- B 1 s 2 2 p 1 3 valence e- Ar 1 s 2 2 p 6 3 s 2 3 p 6 8 valence e- Br 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 7 valence e-

Key Question How does one atom bond to another in a molecule? You will be able to: • create accurate structural formulas using Lewis dot symbols • describe the type of bonding found in molecular substances • explain the chemistry behind the HONC 1234 rule

Prepare for the Activity Lewis dot symbol: A diagram that uses dots to show the valence electrons of a single atom.

Discussion Notes You can use Lewis dot symbols to create Lewis dot structures. A covalent bond is the sharing of a pair of electrons between two nonmetal atoms. Some valence electrons are not involved in bonding. Bonded pair: A pair of electrons that are shared in a covalent bond between two atoms. Lone pair: A pair of valence electrons not involved in bonding within a molecule. The two electrons belong to one atom.

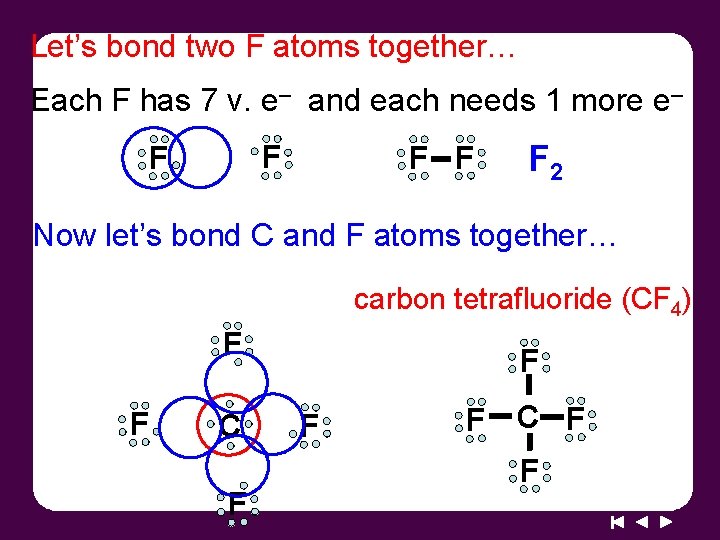
Let’s bond two F atoms together… Each F has 7 v. e– and each needs 1 more e– F F F 2 Now let’s bond C and F atoms together… carbon tetrafluoride (CF 4) F F C F F

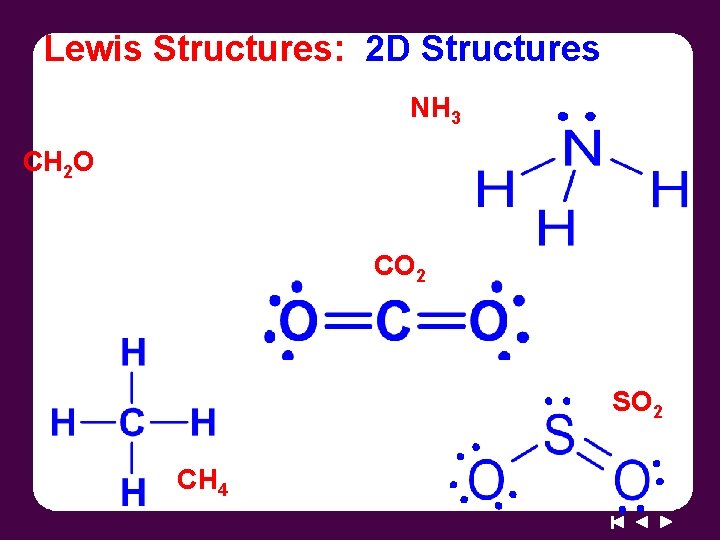
Lewis Structures: 2 D Structures NH 3 CH 2 O CO 2 SO 2 CH 4

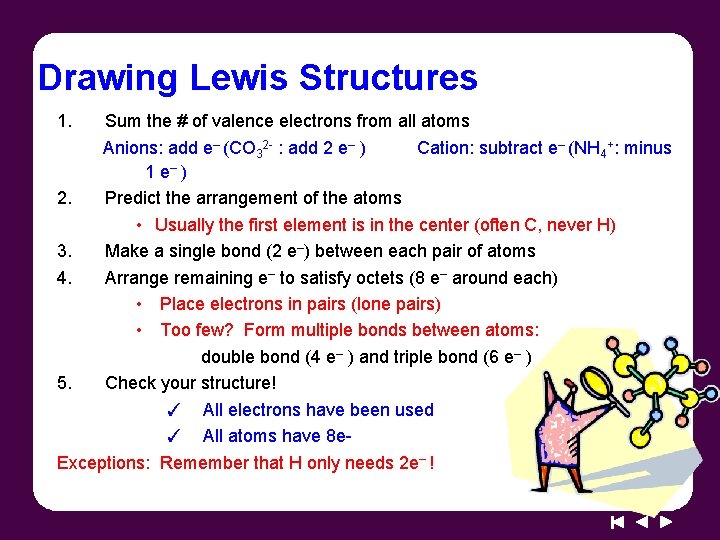
Drawing Lewis Structures 1. Sum the # of valence electrons from all atoms Anions: add e– (CO 32 - : add 2 e– ) Cation: subtract e– (NH 4+: minus 1 e– ) 2. Predict the arrangement of the atoms • Usually the first element is in the center (often C, never H) 3. Make a single bond (2 e–) between each pair of atoms 4. Arrange remaining e– to satisfy octets (8 e– around each) • Place electrons in pairs (lone pairs) • Too few? Form multiple bonds between atoms: double bond (4 e– ) and triple bond (6 e– ) 5. Check your structure! ✓ All electrons have been used ✓ All atoms have 8 e. Exceptions: Remember that H only needs 2 e– !

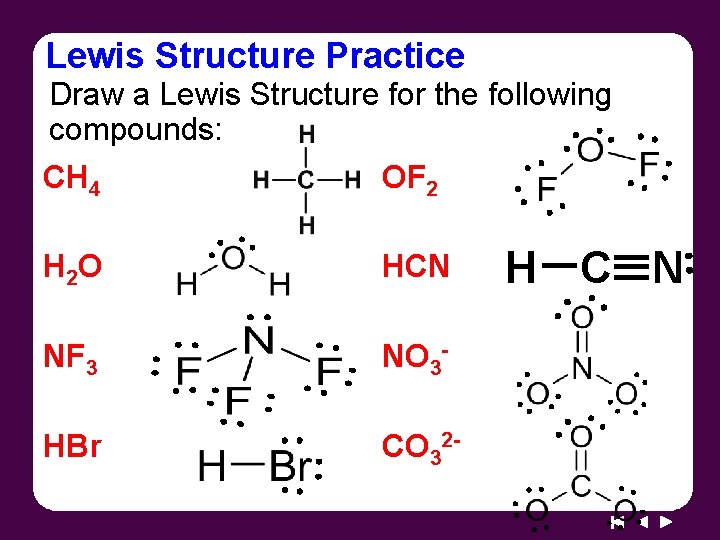
Lewis Structure Practice Draw a Lewis Structure for the following compounds: CH 4 OF 2 H 2 O HCN NF 3 NO 3 - HBr CO 32 - H C N

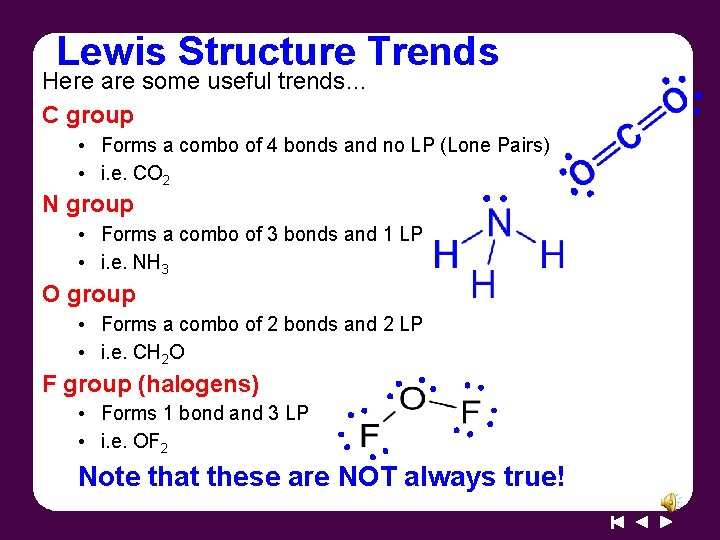
Lewis Structure Trends Here are some useful trends… C group • Forms a combo of 4 bonds and no LP (Lone Pairs) • i. e. CO 2 N group • Forms a combo of 3 bonds and 1 LP • i. e. NH 3 O group • Forms a combo of 2 bonds and 2 LP • i. e. CH 2 O F group (halogens) • Forms 1 bond and 3 LP • i. e. OF 2 Note that these are NOT always true!

Now You Try on your MATS! ~ You can check answers on the keys in the center of your pods ~There are SOME exceptions to the OCTET rule. . . BSF PBr 3 HBr N 2 H 2 C 2 H 5 OH (ethanol) CH 3 OH (methanol) N 2 F 4 NO 2 -1 SF 6 C 2 H 4

Wrap Up How does one atom bond to another in a molecule? • A covalent bond is a bond in which two atoms share a pair of valence electrons. • Lewis dot symbols show the valence electrons in an atom and are used to predict bonding in a molecule.

Wrap Up (cont. ) • In a Lewis dot structure, a pair of electrons that are shared in a covalent bond is called a bonded pair. Pairs of electrons that are not involved in bonding and belong to one atom are referred to as lone pairs. • The HONC 1234 rule indicates how many electrons are available for bonding in atoms of hydrogen, oxygen, nitrogen, and carbon.

Check-In The molecular formula C 4 H 10 O has seven different isomers. Draw the structural formula of one of them. You can use your puzzle pieces to assist you.