Chem A Unit 5 notes EDGENUITY Scientific Notation









































- Slides: 56

Chem A Unit 5 notes EDGENUITY

Scientific Notation A way of writing very large or small numbers as the product of a number, n, between one and ten, and a power of ten, m. n x 10 m 24, 000 = n = 2. 4. m = 4. 0. 000065 Positive Answer is 2. 4 x 104 = n = 6. 5. m = -5. Answer is 6. 5 x 10 -5 exponent is a LARGE number. Negative exponent is a number smaller than one.

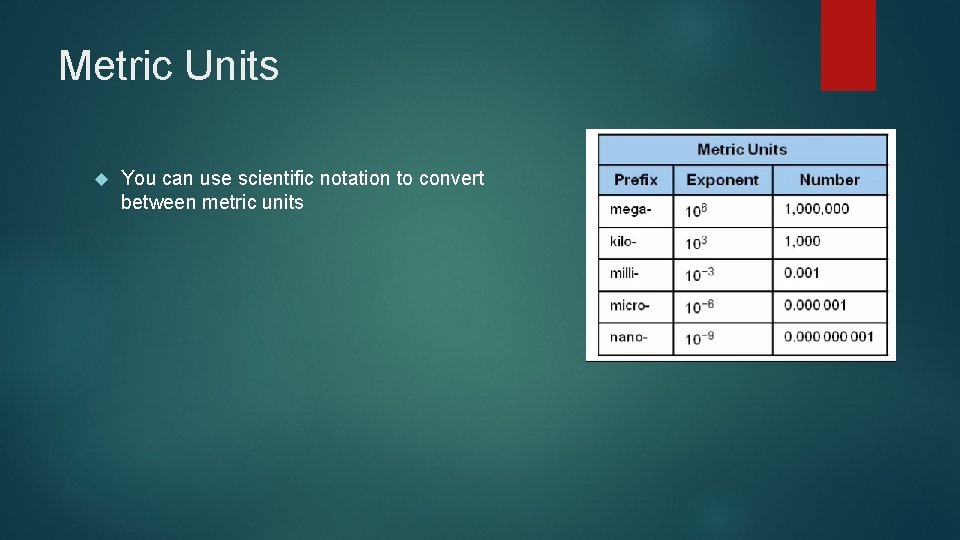
Metric Units You can use scientific notation to convert between metric units

Practice Write 491, 000, 000 m in scientific notation Write 0. 0000914 L in scientific notation 9. 14 x 10 -5 L Write 2. 5 x 105 km in standard notation 4. 91 x 10^11 m. (You can use a carot instead of making the exponent a superscript if that’s easier 250, 000 km Write 91 x 10 -6 g in standard notation 0. 0000091 g

Add and subtract in scientific notation Exponents must be the same in order to add or subtract scientific notation You can also just use a calculator in Chemistry – just make sure you type it into the calculator correctly KHAN VIDEO

Multiply and divide in scientific notation Multiply the numbers between one and ten, then add the exponents Divide the numbers between one and ten, then subtract the exponents Or just use a calculator for Chemistry KHAN VIDEO

Significant figures in measurements Significant figures: the digits that are known for a measurement plus one estimated digit. Abbreviated as sig figs. Example: Mass of 55. 4428 g. This measurement has 6 sig figs. Mass of 55. 44 g. This measurement has 4 sig figs. Mass of moon: 73, 476, 730, 924, 573, 500, 000 kg. This has 15 sig figs. The zeros at the end of the number are not significant. They are place holders

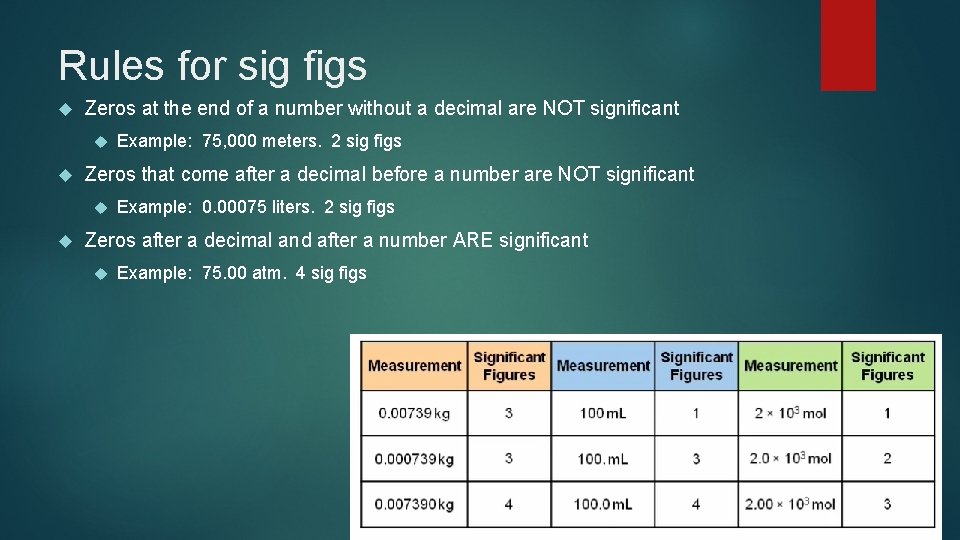
Rules for sig figs Zeros at the end of a number without a decimal are NOT significant Zeros that come after a decimal before a number are NOT significant Example: 75, 000 meters. 2 sig figs Example: 0. 00075 liters. 2 sig figs Zeros after a decimal and after a number ARE significant Example: 75. 00 atm. 4 sig figs

Adding and subtracting with sig figs The result has the same number of decimals as the least precise number 13. 45 meters + 247. 0 meters + 12. 322 meters When you add these on your calculator you get 272. 772 meters. Your answer can’t be more specific than some of your measurements. You must round it to one place past the decimal so it should be 272. 8 meters.

Multiplying and Dividing When you multiply and divide your answer cannot have more sig figs than your measurements. 6. 24 cm x 4. 1 cm x 10. 6 cm = 271. 1904 cm 3 but your answer can only have 2 sig figs so you must round to 270 cm 3

More on sig figs Conversion factors don’t affect the number of sig figs Significant figures only apply to measurements In multistep calculations, the same rules apply KHAN VIDEOS – there are several on this page to choose from

Dimensional Analysis A different name for conversions. VERY useful in Chemistry. We will use D. A. almost all of second semester! You MUST put units on all numbers

Dimensional Analysis pizza example How much will the pizza for the party cost? 6 people at the party. Each person will eat 4 slices. Each pizza has 8 slices. Each pizza costs $15 You 6 people x 4 slices = 24 slices 24 3 can figure this out: slices / 8 slices per pizza = 3 pizzas x $15 per pizza = $45

Now use dimensional analysis to solve

Dimensional Analysis A different name for conversions. VERY useful in Chemistry. We will use D. A. almost all of second semester! You MUST put units on all numbers

Dimensional Analysis Steps q 1. Determine your target – what do you want to know? q 2. Determine what you already know q 3. Set up the problem – make sure your units line up! q 4. Solve the problem – cancel your units! q 5. Check if your answer is reasonable q 6. Sig Fig your final answer – the same number that you started with!

Conversion Practice Conversion factor equals one. It’s just two different ways of expressing the same thing. 60 min = 1 hour. 60 min/1 hour = 1 1 hour/60 min = 1 How many minutes in 5. 5 hours? How many hours in 25 minutes? How many seconds are in an 8 hour workday?

Conversions Practice 0. 056 km to meters 2. 7. 3 mg to grams 3. 94. 5 g to micrograms 1.

Converting with Density Densities are in g/ml or g/cm 3. These can be used as conversion factors: What is the mass of cough syrup that has a volume of 50. 0 cm 3? The density of cough syrup is 0. 950 g/cm 3.

Practice Gold has a density of 19. 3 g/cm 3. What is the density in kilograms per cubic meter? Convert the following using D. A. 8. 2 x 10 -4 micrometers to centimeters 865 cm 3 to liters

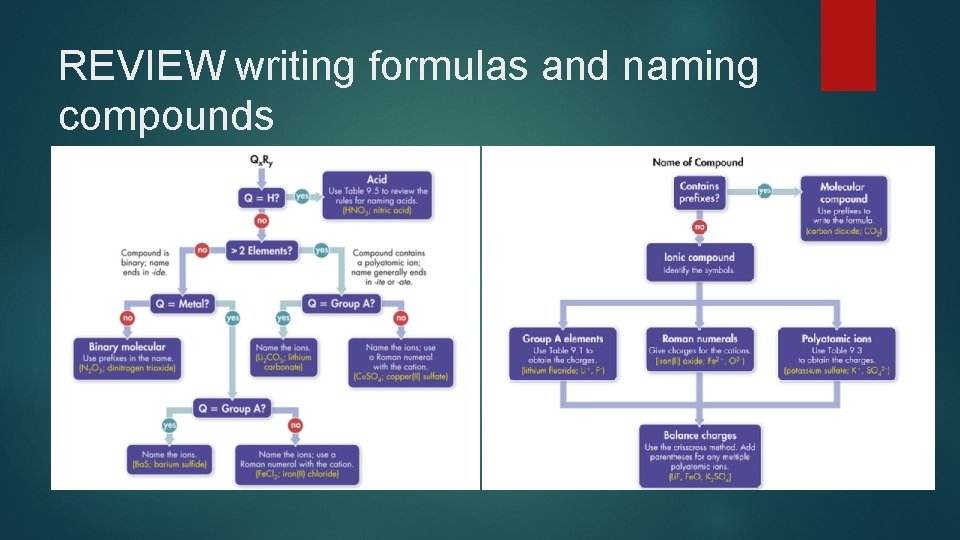
REVIEW writing formulas and naming compounds

The MOLE How many dozen apples do you have if you have 48 apples? What is the mass of 90 apples if 1 dozen of the apples has a mass of 2. 0 kg? Assume 2. 0 kg of apples is 1 dozen and that each apple has 8 seeds. How many apple seeds are in 14 kg of apples?

A mole is another unit for counting things Atoms are VERY small, so we need to have numbers that represent A LOT of them! 1 mole = 6. 02 x 1023 representative particles This 6. 02 x 1023 is Avogadro’s number Representative particle refers to the number of atoms, molecules, or formula units in a substance Conversion factors: 1 mol = 6. 02 x 1023 rep. particles. Use this to solve the problems just like 1 dozen = 12 particles

Converting atoms to moles and moles to atoms How many moles is 2. 80 x 1024 atoms of silicon? How many atoms are in 1. 14 mol of sulfur trioxide (SO 3)? How many Carbon atoms are in 2. 12 mol of propane? How many Hydrogen atoms? Propane is C 3 H 8.

MOLAR MASS Remember atomic masses are listed on the periodic table. The atomic mass of an element expressed in GRAMS is the mass of one mole of the element. This is called Molar Mass. Units are g/mol. Grams per mole. What is the molar mass of Carbon? Hydrogen? Sulfur? You can round to one place past the decimal point.

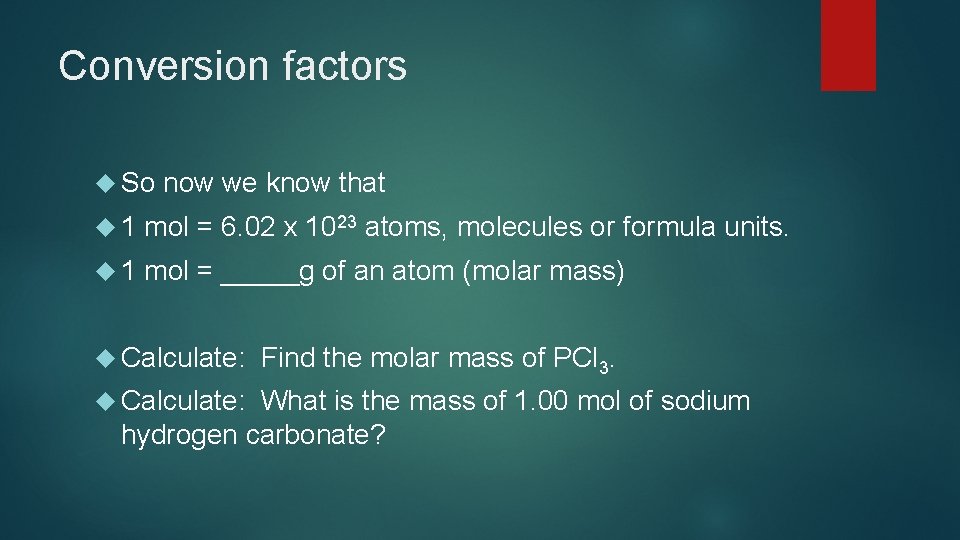
Conversion factors So now we know that 1 mol = 6. 02 x 1023 atoms, molecules or formula units. 1 mol = _____g of an atom (molar mass) Calculate: Find the molar mass of PCl 3. What is the mass of 1. 00 mol of sodium hydrogen carbonate?

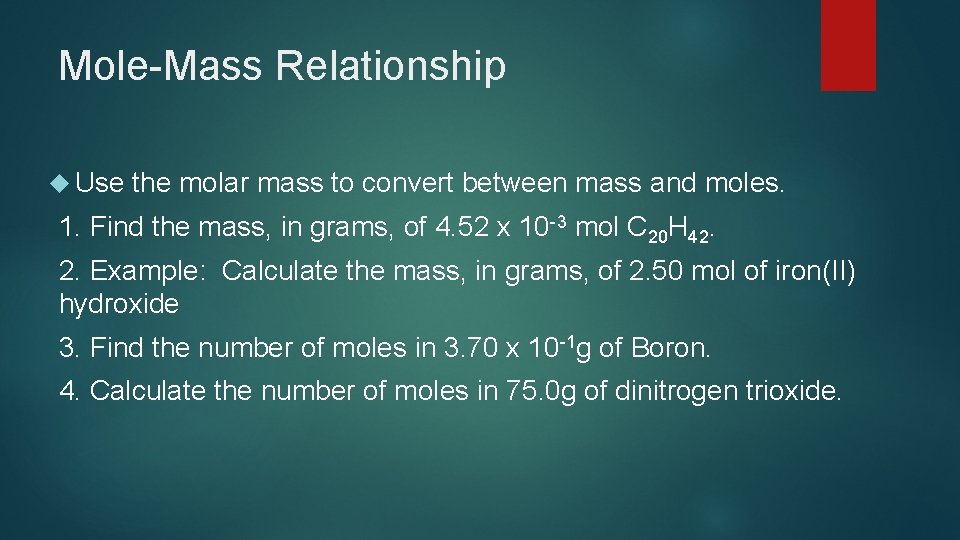
Mole-Mass Relationship Use the molar mass to convert between mass and moles. 1. Find the mass, in grams, of 4. 52 x 10 -3 mol C 20 H 42. 2. Example: Calculate the mass, in grams, of 2. 50 mol of iron(II) hydroxide 3. Find the number of moles in 3. 70 x 10 -1 g of Boron. 4. Calculate the number of moles in 75. 0 g of dinitrogen trioxide.

Stoichiometry This puts EVERYTHING we have learned together! Formulas, reactions, moles, conversions, etc. Units will help! Include units on every number and cancel them to be sure you have set things up correctly! You must show all of you work!! need to use dimensional analysis for these problems!

Stoichiometry using a sandwich 1 PB + 1 jelly + 2 bread ------ 1 sandwich If I want to make 4 sandwiches, how much bread do I need? If I have 17 Jellys, how many sandwiches can I make?

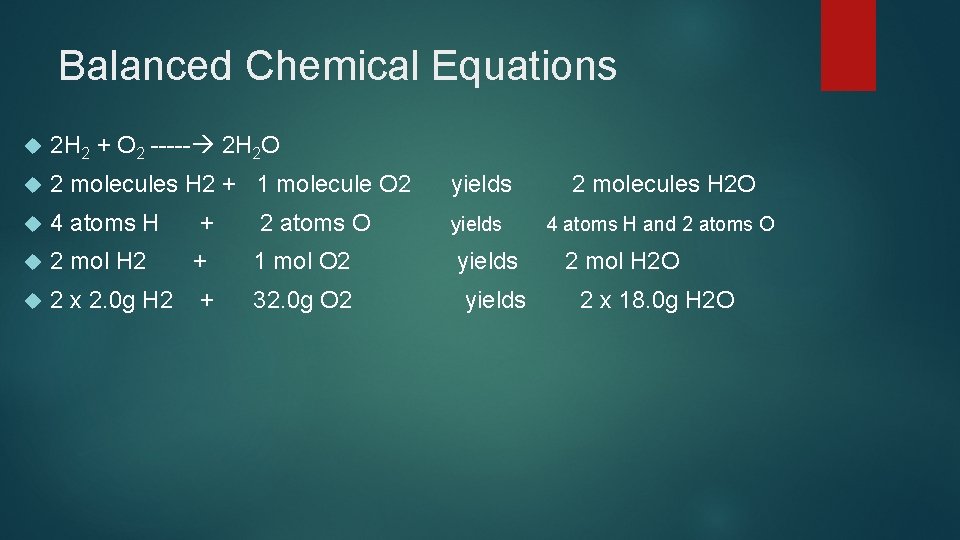
Balanced Chemical Equations 2 H 2 + O 2 ----- 2 H 2 O 2 molecules H 2 + 1 molecule O 2 yields 2 molecules H 2 O 4 atoms H + 2 atoms O yields 4 atoms H and 2 atoms O 2 mol H 2 + 1 mol O 2 2 x 2. 0 g H 2 + 32. 0 g O 2 yields 2 mol H 2 O 2 x 18. 0 g H 2 O

Mole Ratios 4 Al + 3 O 2 ----- 2 Al 2 O 3 4 mol Al: 3 mol O 2 4 mol Al: 2 mol Al 2 O 3 3 mol O 2: 2 mol Al 2 O 3 Use these as conversion factors!!

Steps in solving Usually convert to moles first Use mole ratios to go from one substance to another Convert from moles to whatever you are trying to find: mass, volume, particles.

More steps Moles to mass: Molar mass Moles to particles: 6. 02 x 1023 particles = 1 mol

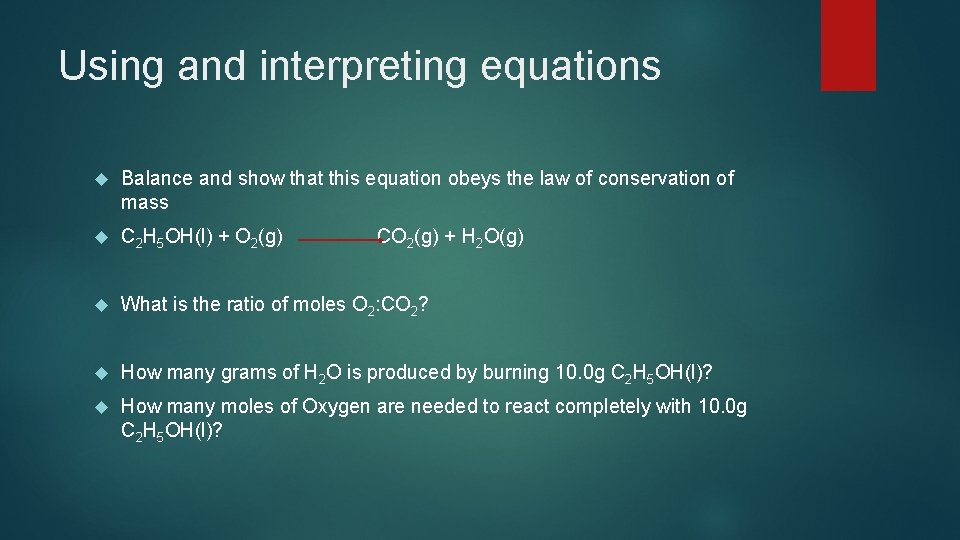
Using and interpreting equations Balance and show that this equation obeys the law of conservation of mass C 2 H 5 OH(l) + O 2(g) What is the ratio of moles O 2: CO 2? How many grams of H 2 O is produced by burning 10. 0 g C 2 H 5 OH(l)? How many moles of Oxygen are needed to react completely with 10. 0 g C 2 H 5 OH(l)? CO 2(g) + H 2 O(g)

Limiting reagent If you don’t have enough of one reactant, the amount of product that can be formed is limited. To find which reactant is the limiting reagent, find the amount of moles of each reactant. Convert moles of one to moles of another. If the amount needed to react is less, than it is the limiting reactant. If the amount is more, the other is limiting. Excess reactant: The reactant that there is enough of or is not fully consumed in a reaction

Limiting reagent using a sandwich 1 PB + 1 jelly + 2 bread ------ 1 sandwich How many sandwiches can I make if I have 5 jellys, 3 PB and 8 breads? Which is the limiting reagent? Which reactant is in excess?

Limiting reactant example If 2. 70 mol C 2 H 4 reacts with 6. 30 mol O 2, identify the limiting reagent. (It’s a combustion reaction) 1. Write balanced equation. 2. Convert from mass to moles for each reactant 3. Use moles of one to convert to moles of another and compare it to moles given. OR convert to amount of product for both reactants and see which one produces less.

Theoretical yield How much product you would get in a perfect world. Simply use stoichiometry to go from mass of reactant to mass of product to calculate it.

Percent yield = actual yield/theoretical yield x 100

Practice Consider the following reaction: 3 NH 4 NO 3 + Na 3 PO 4 → (NH 4)3 PO 4 + 3 Na. NO 3 Which of the reagents is the limiting reagent? What is the maximum amount of each product that can be formed? How much of the other reagent is left over after the reaction is complete? We started with 30. 0 grams of ammonium nitrate and 50. 0 grams of sodium phosphate.

Theoretical/actual yield practice Balance this equation and state which of the six types of reaction is taking place: ____ Mg + ____ HNO 3 → ____ Mg(NO 3)2 + ____ H 2. reaction: 2) If I start this reaction with 40. grams of magnesium and an excess of nitric acid, how many grams of hydrogen gas will I produce? 3) If 1. 70 grams of hydrogen is actually produced, what was my percent yield of hydrogen? Type of

More Stoichiometry practice KHAN Academy You can get extra credit if you do the practice/quizzes and webmail me screen shots of your scores! 1. Using the following equation: 2 Na. OH + H 2 SO 4 → 2 H 2 O + Na 2 SO 4 How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid? 2. Using the following equation: Pb(SO 4)2 + 4 Li. NO 3 → Pb(NO 3)4 + 2 Li 2 SO 4 How many grams of lithium nitrate will be needed to make 250 grams of lithium sulfate, assuming that you have an adequate amount of lead (IV) sulfate to do the reaction?

Balance the following equations 1) ___ N 2 + ___ F 2 → ___ NF 3 2) ___ C 6 H 10 + ___ O 2 → ___ CO 2 + ___ H 2 O 3) ___ HBr + ___ KHCO 3 → ___ H 2 O + ___ KBr + ___ CO 2 4) ___ Ga. Br 3 + ___ Na 2 SO 3 → ___ Ga 2(SO 3)3 + ___ Na. Br 5) ___ Sn. O + ___ NF 3 → ___ Sn. F 2 + ___ N 2 O 3

Using number 2 from the previous slide, answer the following: 1. If I do this reaction with 35 grams of C 6 H 10 and 45 grams of oxygen, how many grams of carbon dioxide will be formed? 2. What is the limiting reagent for problem 6? ______ 3. How much of the excess reagent is left over after the reaction from problem 6 is finished? 4. If 35 grams of carbon dioxide are actually formed from the reaction in problem 6, what is the percent yield of this reaction?

Measures of Concentration - molarity Concentration: Amount of solute divided by the amount of solvent. More solute dissolved in a given volume yields a higher concentration Less solute dissolved in a given volume yields a lower concentration. Unsaturated solution has a lower concentration than a saturated solution which has a lower concentration than a super saturated solution

Expressing concentration Molarity – the concentration of a solution expressed as the number of moles of solute per liter of solution. M = mol/L Represented with brackets: [H+] = concentration of H+ in mol/L PHET TUTORIAL Drag the concentration meter (the purple circle attached to a wire) into the water and select the solute cobalt (II) chloride (Co. Cl 2). 2. Move the shaker continuously to add cobalt (II) chloride until the concentration is 4. 330 mol/L. How would you classify this solution? 3. What happened to the color of the solution as you added more of the ionic compound? 4. Tap the faucet to add another 0. 5 L of water, bringing the total volume of the solution to 1 L. When you doubled the water volume, what happened to the concentration? How would you now classify this solution? Complete a similar process for two other ionic compounds. Experiment with different amounts of the ionic solute and the water solvent to observe the changing concentrations.

Calculating molarity You M need to know moles and liters to calculate molarity = mol solute/liters solution Find the molarity of a solution of Na. Cl if 500. 0 m. L of solution contains 11. 6 g of Na. Cl. Find molar mass of Na. Cl: 58. 44 g Convert 11. 6 g to moles of Na. Cl: 11. 6 g x (1 mol/58. 44 g) = 0. 198 mol Convert Find 500. 0 m. L to Liters: 500. 0 m. L x (1 L/1000 m. L) = 0. 5 L molarity: M = 0. 198 mol/0. 5 L = 0. 396 M

Using molarity to calculate other quantities M = mol/L. Use algebra to rearrange the equation OR use dimensional analysis and use molarity as a conversion factor Calculate moles of solute: Multiply molarity by volume. M x L = mol Calculate volume of solution: L = mol/M

Practice How many moles of 0. 100 M Na. OH are present in 200. 0 m. L of solution? How many grams of Na. OH are in this solution. 1. 2. Calculate the number of moles of the solute Convert the moles to grams using the molar mass of Na. OH

A solution of HCl has a molarity of 4. 00 M HCl. If a reaction requires 0. 73 g of HCl, what volume of the solution should be used? Calculate the moles of HCl needed: 0. 73 g x (1 mol/36. 46 g) = 0. 020 mol HCl. Calculate the volume: 0. 020 mol x (1 L/4. 00 mol) x (1000 m. L/1 L) = 5. 0 m. L of the HCL solution

Stoichiometry involving molarity Use the molarity as a conversion factor in the dimensional analysis problems How many m. L of a 0. 500 M Na. OH solution are required to completely react with the HCl in 100. 0 m. L of a 0. 100 M HCl solution? 100. 0 m. L x (1 L/1000 m. L) x (0. 100 mol HCl/1 L) x (1 mol Na. OH/1 mol HCl) x (1 L/0. 500 mol Na. OH) x (1000 m. L/1 L) = Check and cancel units, then do the math You should get 20. 0 m. L Na. OH

Dilution – adding more solvent to a solution to decrease the concentration Dilute solutions are weaker, or less concentrated than concentrated solutions. They have a smaller amount of solute

How can we dilute a solution Add more solvent Remove some of the original solution and mix with water

Concentrated solutions can be diluted by adding solvent. Dilution equation: M 1 V 1 = M 2 V 2 OR Mi. Vi = Mf. Vf A chemist adds 100. 0 m. L of concentrated (12 M) HCl to 100. 0 m. L of water. What is the new solution’s concentration? M 1 = 12 M, V 1 = 100. 0 m. L, V 2 = 200. 0 m. L, M 2 = ? Plug into the equation: (12 M x 100. 0 m. L)/200. 0 m. L = M 2 = 6 M

You must dilute a 6. 00 M HCl solution to a concentration of 3. 00 M. You need 40. 0 m. L of the 3. 00 M HCl solution. What volume of 6. 00 M HCl does he need? How much water does he need to add? M 1 V 1 M 1 = M 2 V 2 = 6. 00 M, V 1 = ? , M 2 = 3. 00 M, V 2 = 40. 0 m. L (3. 00 M x 40. 0 m. L)/6. 00 M = V 1 = 20. 0 m. L of the 6. 0 M HCl is needed. He also needs to add 20. 0 m. L of water to get the solution to have a volume of 40. 0 m. L.

Stock solutions Stock solution – a concentrated solution of a common reagent used to prepare more diluted solutions to be used in specific reactions Any lower concentration can be produced from stock solution