CHEM 7010 Macromolecular Synthesis 2 nd Introduction to















- Slides: 43

CHEM 7010 Macromolecular Synthesis 2 nd Introduction to Polymer Synthesis: General Aspects of Polymer Structures on Polymer Properties 2011

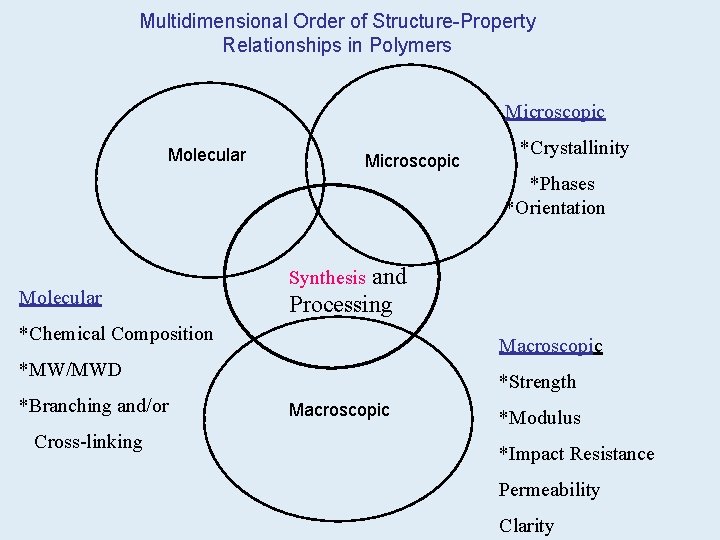
Multidimensional Order of Structure-Property Relationships in Polymers Microscopic Molecular Microscopic *Crystallinity *Phases *Orientation Molecular and Processing Synthesis *Chemical Composition Macroscopic *MW/MWD *Branching and/or Cross-linking *Strength Macroscopic *Modulus *Impact Resistance Permeability Clarity

IMPACT OF MOLECULAR WEIGHT ON MATERIAL PROPERTIES Increasing Degree of Polymerization, DP

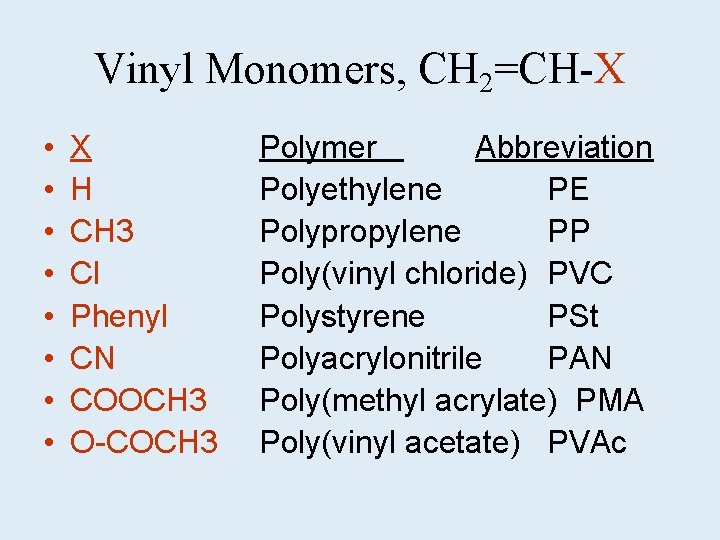
Vinyl Monomers, CH 2=CH-X • • X H CH 3 Cl Phenyl CN COOCH 3 O-COCH 3 Polymer Abbreviation Polyethylene PE Polypropylene PP Poly(vinyl chloride) PVC Polystyrene PSt Polyacrylonitrile PAN Poly(methyl acrylate) PMA Poly(vinyl acetate) PVAc

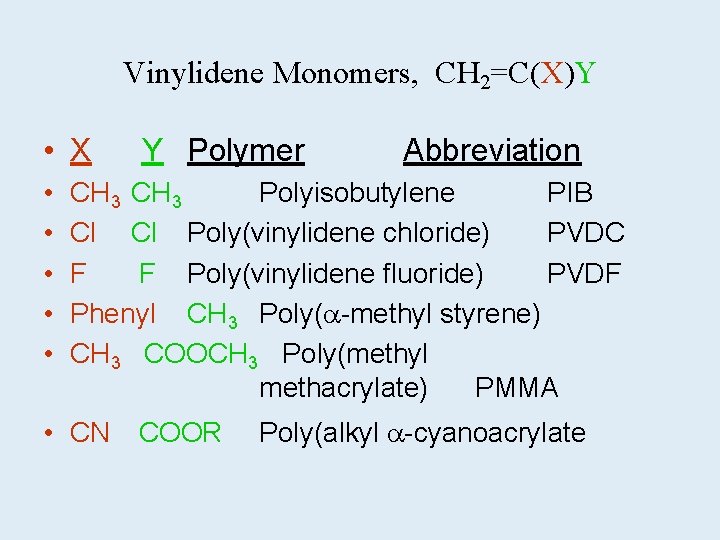
Vinylidene Monomers, CH 2=C(X)Y • X Y Polymer • • • Abbreviation CH 3 Polyisobutylene PIB Cl Poly(vinylidene chloride) PVDC F F Poly(vinylidene fluoride) PVDF Phenyl CH 3 Poly( -methyl styrene) CH 3 COOCH 3 Poly(methyl methacrylate) PMMA • CN COOR Poly(alkyl -cyanoacrylate

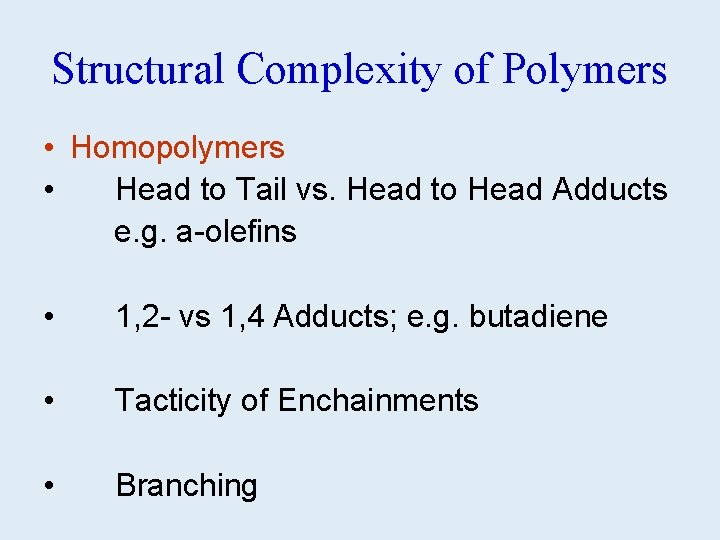
Structural Complexity of Polymers • Homopolymers • Head to Tail vs. Head to Head Adducts e. g. a-olefins • 1, 2 - vs 1, 4 Adducts; e. g. butadiene • Tacticity of Enchainments • Branching

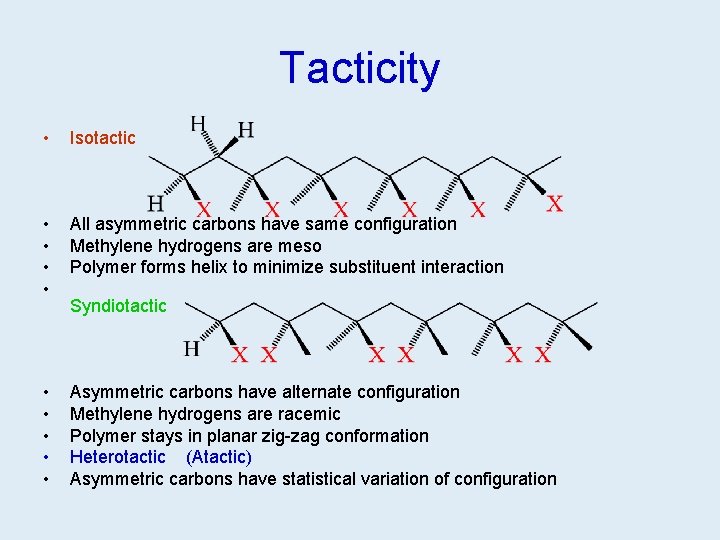
Tacticity • Isotactic • • All asymmetric carbons have same configuration Methylene hydrogens are meso Polymer forms helix to minimize substituent interaction • • • Asymmetric carbons have alternate configuration Methylene hydrogens are racemic Polymer stays in planar zig-zag conformation Heterotactic (Atactic) Asymmetric carbons have statistical variation of configuration Syndiotactic

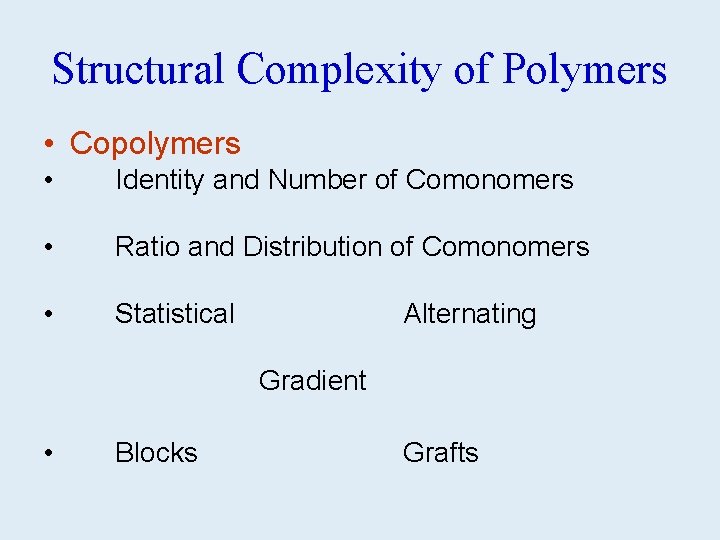
Structural Complexity of Polymers • Copolymers • Identity and Number of Comonomers • Ratio and Distribution of Comonomers • Statistical • Blocks Alternating Gradient Grafts

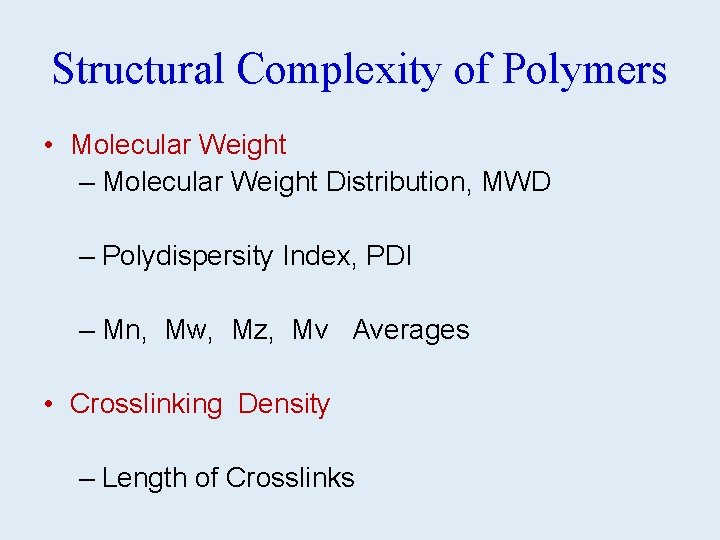
Structural Complexity of Polymers • Molecular Weight – Molecular Weight Distribution, MWD – Polydispersity Index, PDI – Mn, Mw, Mz, Mv Averages • Crosslinking Density – Length of Crosslinks

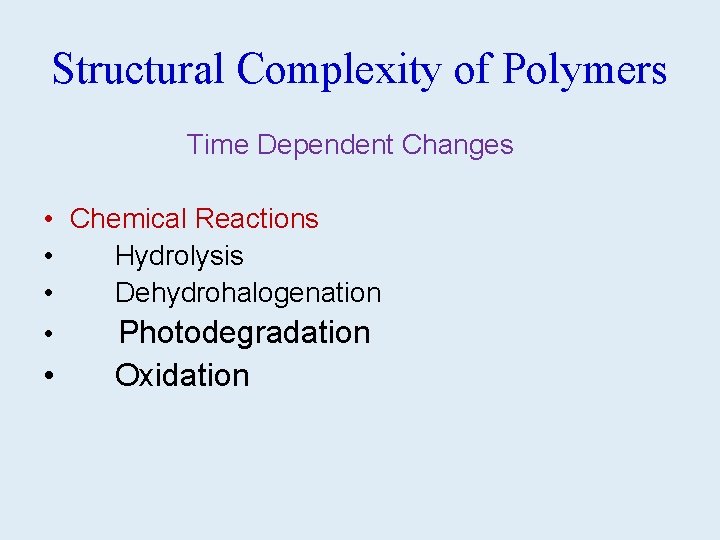
Structural Complexity of Polymers Time Dependent Changes • Chemical Reactions • Hydrolysis • Dehydrohalogenation • Photodegradation • Oxidation

Structural Complexity of Polymers • Thermal Degradation • Processing • Aging • Crystallization • Changes in Polymorphism • Weathering-- Combination of Above • Plasticizer Loss -- Imbrittlement

Microscopic Properties (Intermolecular Interactions) • Morphology • Chain entanglement –amorphous • Chain ordering--liquid crystalline • Crystallinity • Phase separations (microdomains)

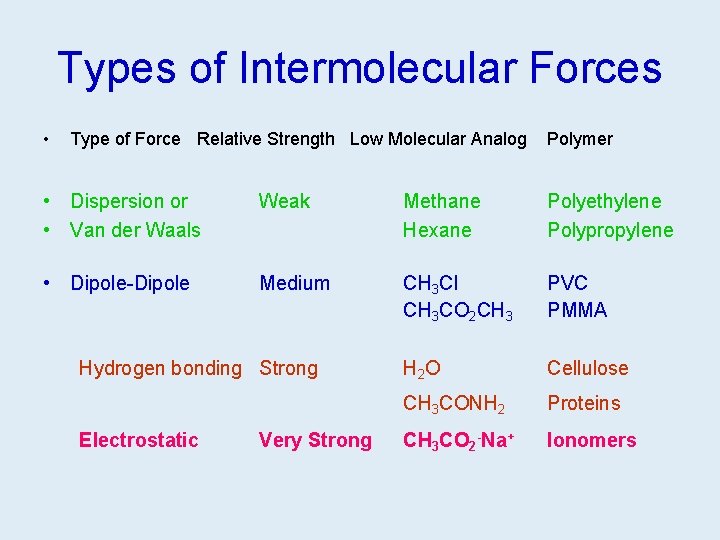
Types of Intermolecular Forces • Type of Force Relative Strength Low Molecular Analog Polymer • Dispersion or • Van der Waals Weak • Dipole-Dipole Medium Hydrogen bonding Strong Electrostatic Very Strong Methane Hexane Polyethylene Polypropylene CH 3 Cl CH 3 CO 2 CH 3 PVC PMMA H 2 O Cellulose CH 3 CONH 2 Proteins CH 3 CO 2 -Na+ Ionomers

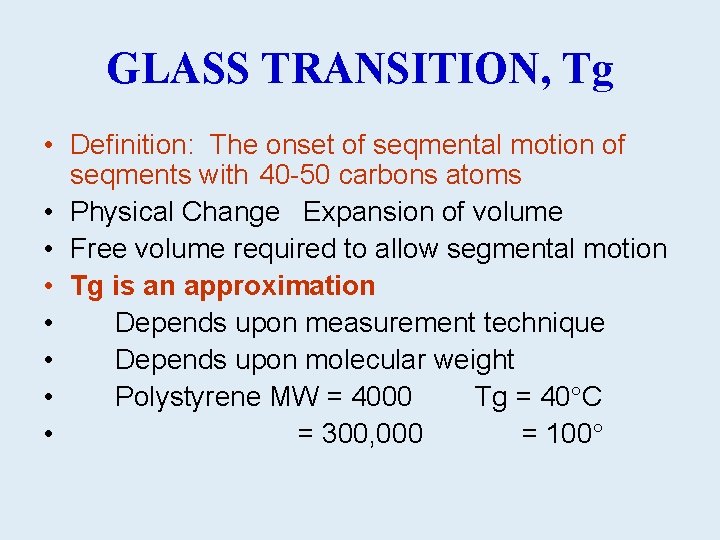
GLASS TRANSITION, Tg • Definition: The onset of seqmental motion of seqments with 40 -50 carbons atoms • Physical Change Expansion of volume • Free volume required to allow segmental motion • Tg is an approximation • Depends upon measurement technique • Depends upon molecular weight • Polystyrene MW = 4000 Tg = 40 C • = 300, 000 = 100

GLASS TRANSITION, Tg • Properties Affected • Specific Volume / Density • Specific Heat, Cp • Refractive Index • Modulus • Dielectric Constant • Permeability

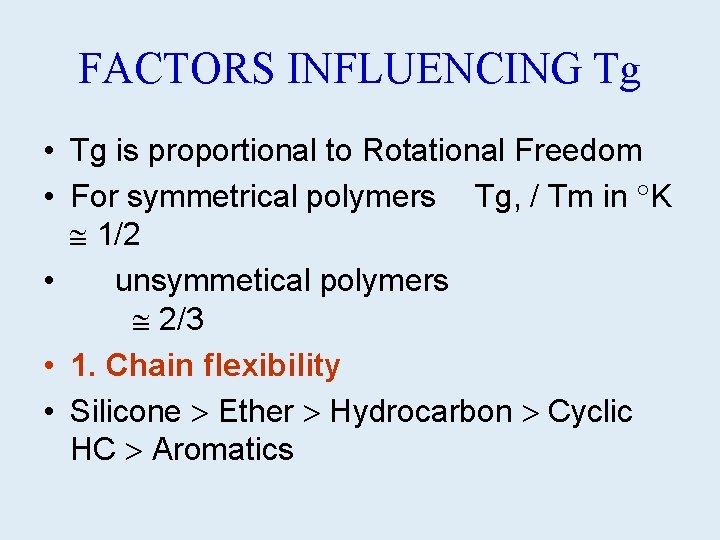
FACTORS INFLUENCING Tg • Tg is proportional to Rotational Freedom • For symmetrical polymers Tg, / Tm in K 1/2 • unsymmetical polymers 2/3 • 1. Chain flexibility • Silicone Ether Hydrocarbon Cyclic HC Aromatics

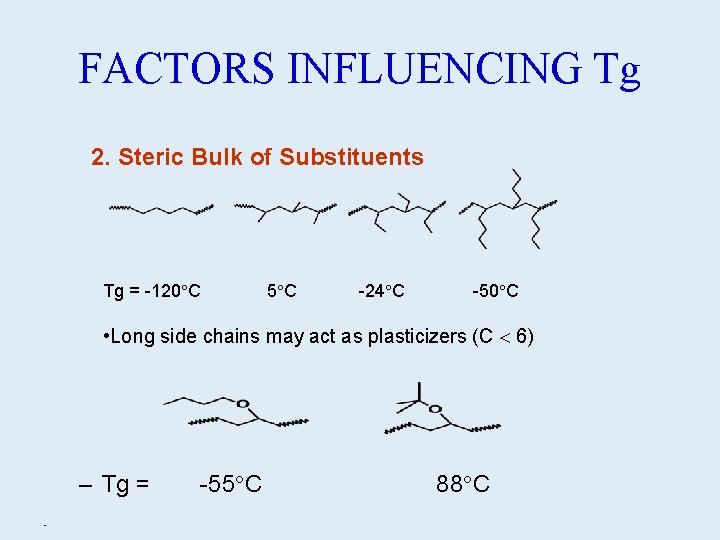
FACTORS INFLUENCING Tg 2. Steric Bulk of Substituents Tg = -120 C 5 C -24 C -50 C • Long side chains may act as plasticizers (C 6) – Tg = • -55 C 88 C

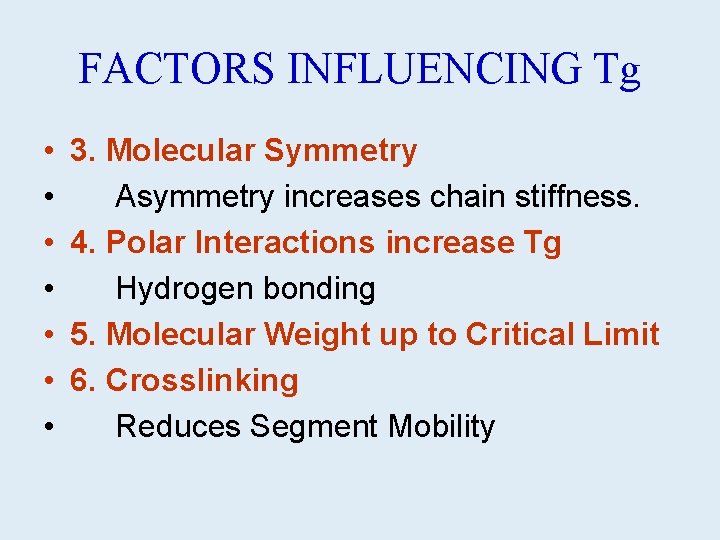
FACTORS INFLUENCING Tg • • 3. Molecular Symmetry Asymmetry increases chain stiffness. 4. Polar Interactions increase Tg Hydrogen bonding 5. Molecular Weight up to Critical Limit 6. Crosslinking Reduces Segment Mobility

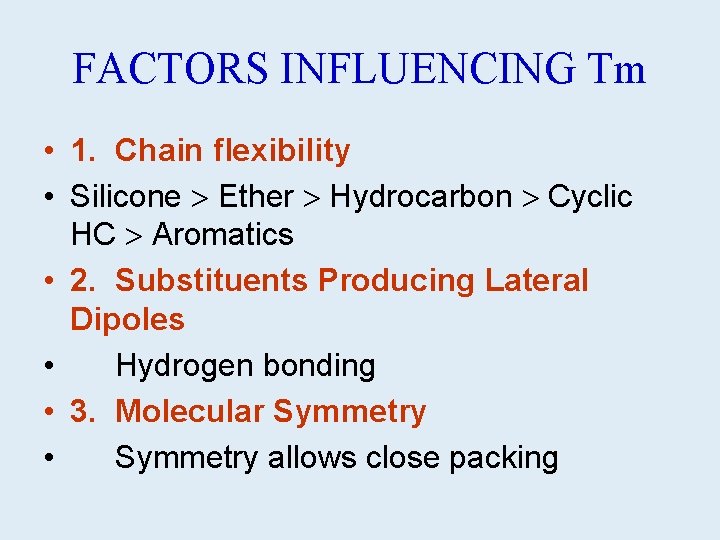
FACTORS INFLUENCING Tm • 1. Chain flexibility • Silicone Ether Hydrocarbon Cyclic HC Aromatics • 2. Substituents Producing Lateral Dipoles • Hydrogen bonding • 3. Molecular Symmetry • Symmetry allows close packing

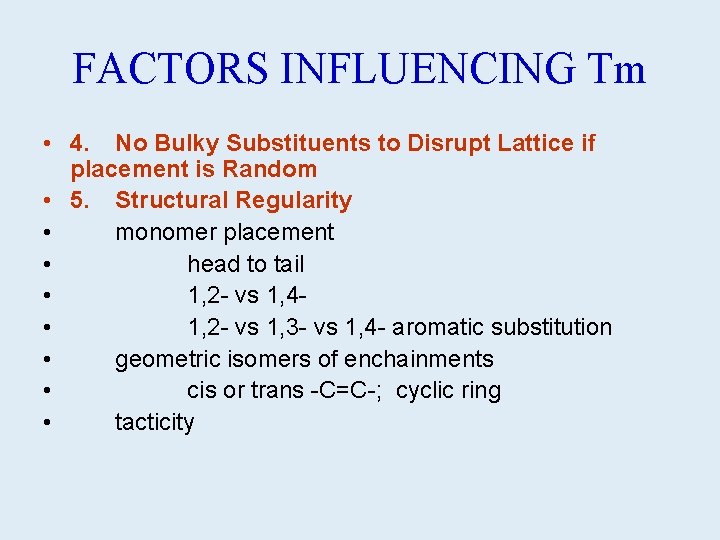
FACTORS INFLUENCING Tm • 4. No Bulky Substituents to Disrupt Lattice if placement is Random • 5. Structural Regularity • monomer placement • head to tail • 1, 2 - vs 1, 4 - • 1, 2 - vs 1, 3 - vs 1, 4 - aromatic substitution • geometric isomers of enchainments • cis or trans -C=C-; cyclic ring • tacticity

FACTORS REQUIRED TO PROMOTE CRYSTALLIZATION • Thermodynamic • 1. Symmetrical chains which allow regular close packing in crystallite • 2. Functional groups which encourage strong intermolecular attraction to stabilize ordered alignment.

FACTORS REQUIRED TO PROMOTE CRYSTALLIZATION • Kinetic • 1. Sufficient mobility to allow chain disentanglement and ultimate alignment • Optimum range for mobility • Tm -10 Tg + 30 • at Tm segmental motion too high • at Tg viscosity too high • 2. Concentration of nuclei • concentration of nucleating agents • thermal history of sample

Macroscopic Properties (Physical Behavior) • Tensile and/or Compressive Strength • Elasticity • Toughness • Thermal Stability • Flammability and Flame Resistance • Degradability • Solvent Resistance • Permeability • Ductility (Melt Flow)

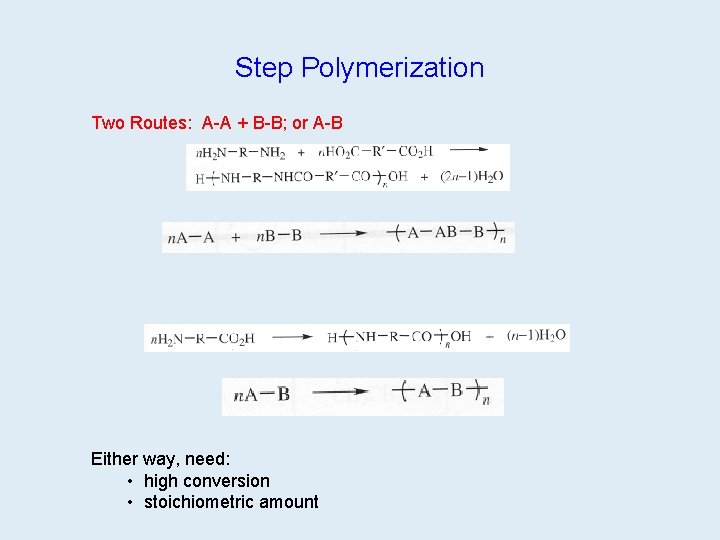
Step Polymerization (Polycondensation) After many repetitions: Polyethyleneterephthalate (PET)

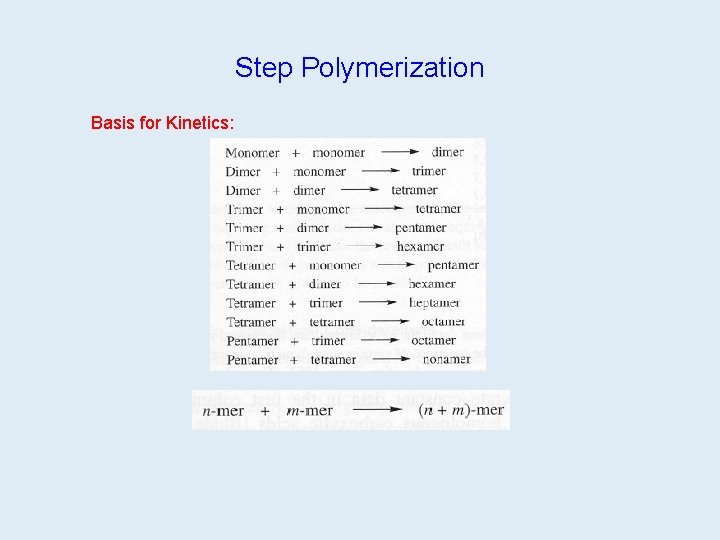
Step Polymerization Two Routes: A-A + B-B; or A-B 1 on 39 1 on 40 2 on 39 2 on 40 Either way, need: • high conversion • stoichiometric amount

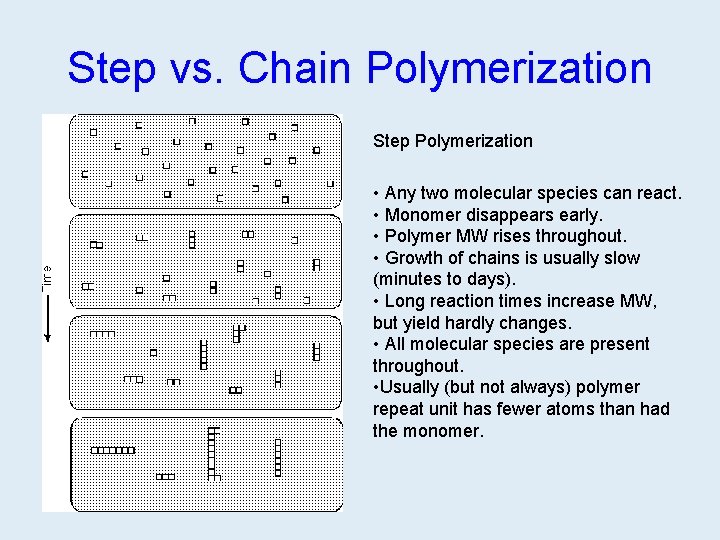
Step vs. Chain Polymerization Step Polymerization • Any two molecular species can react. • Monomer disappears early. • Polymer MW rises throughout. • Growth of chains is usually slow (minutes to days). • Long reaction times increase MW, but yield hardly changes. • All molecular species are present throughout. • Usually (but not always) polymer repeat unit has fewer atoms than had the monomer.

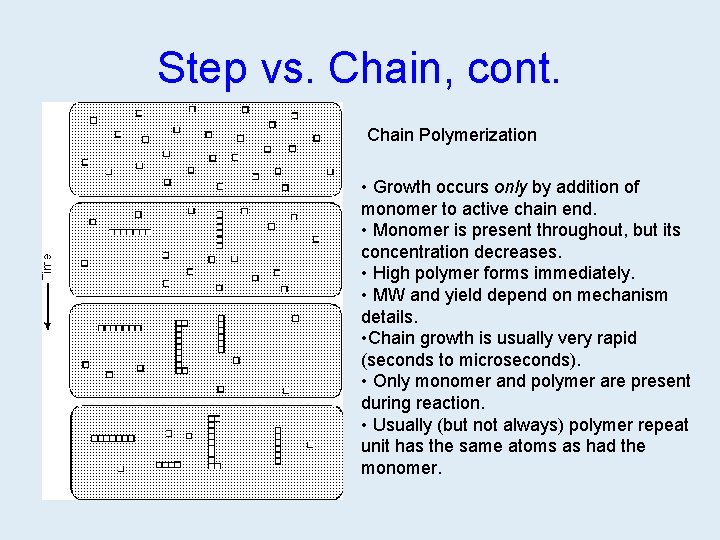
Step vs. Chain, cont. Chain Polymerization • Growth occurs only by addition of monomer to active chain end. • Monomer is present throughout, but its concentration decreases. • High polymer forms immediately. • MW and yield depend on mechanism details. • Chain growth is usually very rapid (seconds to microseconds). • Only monomer and polymer are present during reaction. • Usually (but not always) polymer repeat unit has the same atoms as had the monomer.

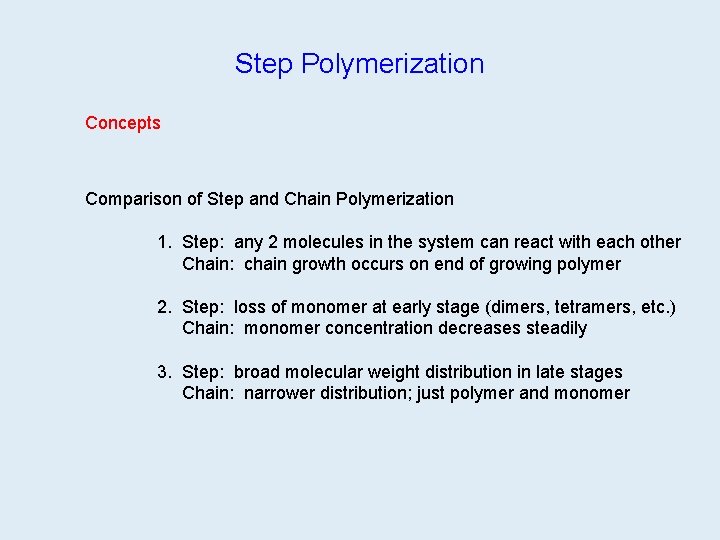
Step Polymerization Concepts Comparison of Step and Chain Polymerization 1. Step: any 2 molecules in the system can react with each other Chain: chain growth occurs on end of growing polymer 2. Step: loss of monomer at early stage (dimers, tetramers, etc. ) Chain: monomer concentration decreases steadily 3. Step: broad molecular weight distribution in late stages Chain: narrower distribution; just polymer and monomer

Step Polymerization Basis for Kinetics (2 -1 a): 3 on 40 4 on 40 5 on 40 6 on 40

Step Polymerization Basis for Kinetics: 1 on 41 2 on 41

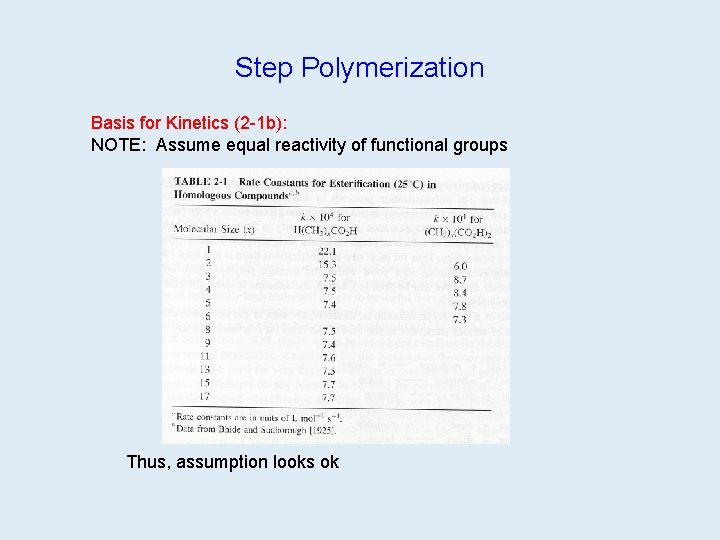
Step Polymerization Basis for Kinetics (2 -1 b): NOTE: Assume equal reactivity of functional groups table 2. 1 on 42 Thus, assumption looks ok

Step Polymerization Kinetics (2. 2) Example: diacid + diol Mechanism (acid catalyzed): 2 on 44 1 on 45 2 on 45

Chain Polymerization Concepts General Mechanism Initiation Propagation

Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 a. Comparison of Chain and Step Polymerizations Chain: a. Rapid high mw b. Snapshot: monomer, high poly, growing chains c. MW does not change with time Step: a. Monomer gone fast, get dimer, trimer, etc. b. MW increases with time c. No high mw until end

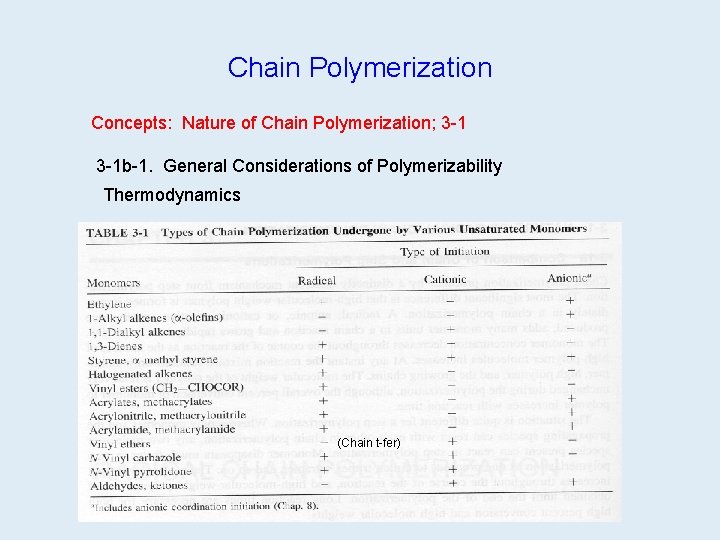
Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 b-1. General Considerations of Polymerizability Thermodynamics (Chain t-fer)

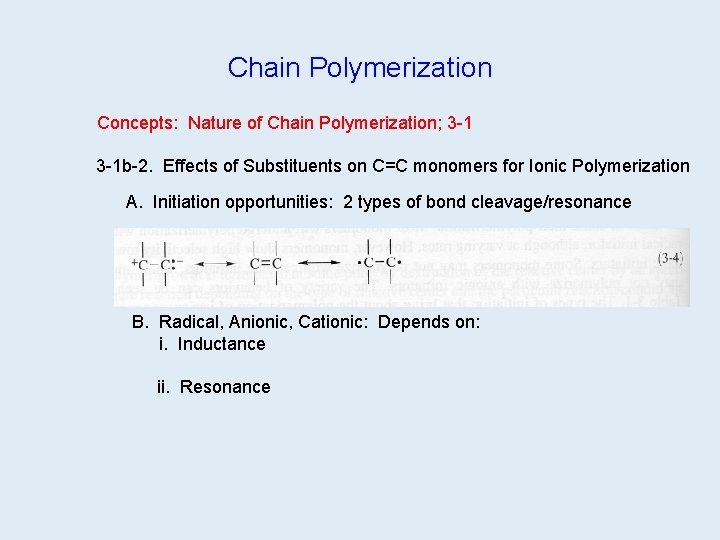
Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 b-2. Effects of Substituents on C=C monomers for Ionic Polymerization A. Initiation opportunities: 2 types of bond cleavage/resonance B. Radical, Anionic, Cationic: Depends on: i. Inductance ii. Resonance

Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 b-2. Effects of Substituents on C=C monomers for Ionic Polymerization C. Donating Groups i. Inductance ii. Resonance e. g. Polym of vinyl ether Good for cationic Example of resonance stabilization of cation by Delocalization of of positive charge. If oxygen not there, no stabilization

Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 b-2. Effects of Substituents on C=C monomers for Ionic Polymerization C. Donating Groups ii. Resonance e. g. Polym of Styrene

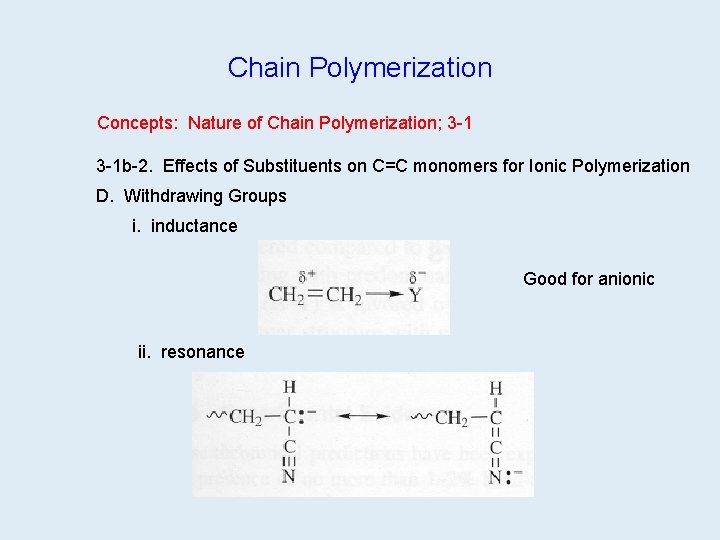
Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 b-2. Effects of Substituents on C=C monomers for Ionic Polymerization D. Withdrawing Groups i. inductance Good for anionic ii. resonance

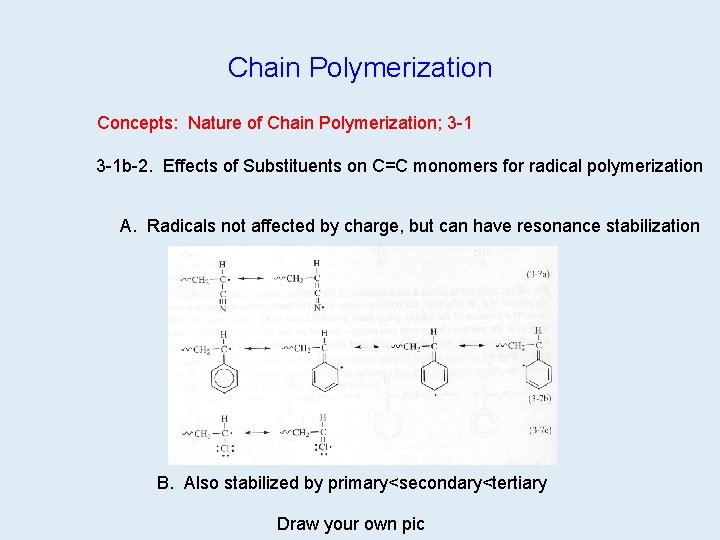
Chain Polymerization Concepts: Nature of Chain Polymerization; 3 -1 b-2. Effects of Substituents on C=C monomers for radical polymerization A. Radicals not affected by charge, but can have resonance stabilization B. Also stabilized by primary<secondary<tertiary Draw your own pic

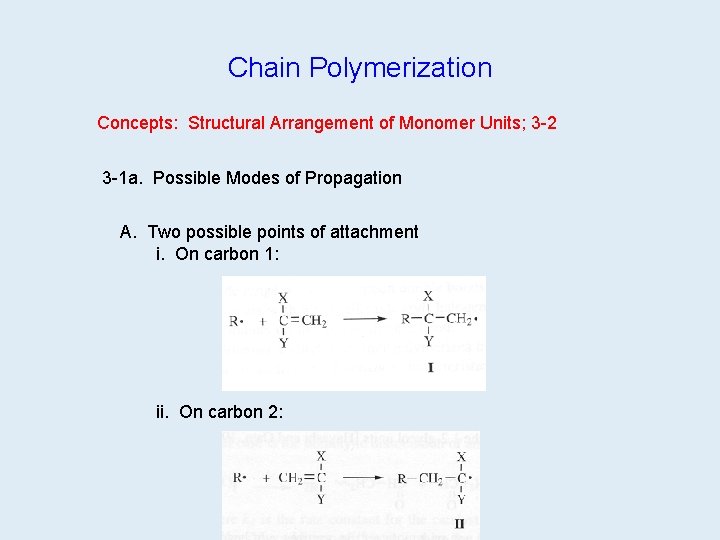
Chain Polymerization Concepts: Structural Arrangement of Monomer Units; 3 -2 3 -1 a. Possible Modes of Propagation A. Two possible points of attachment i. On carbon 1: ii. On carbon 2:

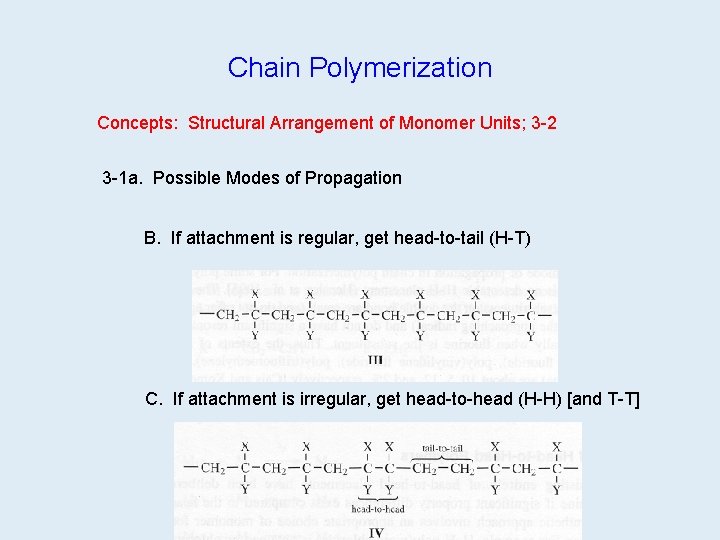
Chain Polymerization Concepts: Structural Arrangement of Monomer Units; 3 -2 3 -1 a. Possible Modes of Propagation B. If attachment is regular, get head-to-tail (H-T) C. If attachment is irregular, get head-to-head (H-H) [and T-T]

Chain Polymerization Concepts: Structural Arrangement of Monomer Units; 3 -2 3 -1 a. Possible Modes of Propagation Usually only 1 -2% H-H. How do we know? A. Chemical Methods B. NMR Methods (we’ll check this out in the Spectroscopy section)
 Iannonechem
Iannonechem Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Imf chem
Imf chem Chem 130 final exam
Chem 130 final exam Alkanes formula
Alkanes formula Kmt chem
Kmt chem Chemistry january 2018 answers
Chemistry january 2018 answers Introduction to spectrophotometry
Introduction to spectrophotometry Chem 1020
Chem 1020 Ion induced dipole example
Ion induced dipole example Chemistry law hazard pay
Chemistry law hazard pay Gen chem review for ochem
Gen chem review for ochem Chem 109
Chem 109 Chem feed
Chem feed Chemikalienportal
Chemikalienportal Amy gottfried umich
Amy gottfried umich Chempro 100i
Chempro 100i Chem 253
Chem 253 Equations for acids and bases chem worksheet 19-1
Equations for acids and bases chem worksheet 19-1 Ka acid base
Ka acid base Thermochemistry vocabulary
Thermochemistry vocabulary Magnesium pes spectrum
Magnesium pes spectrum Ap chemistry kinetics
Ap chemistry kinetics Spectroscopy ap chem
Spectroscopy ap chem Ap chemistry equilibrium review
Ap chemistry equilibrium review Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Ap chemistry entropy and free energy
Ap chemistry entropy and free energy 2017 ap chemistry practice exam
2017 ap chemistry practice exam Ario acronym chemistry
Ario acronym chemistry Chem quiz.net
Chem quiz.net Model chem lab
Model chem lab Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Bó thon bó chêm
Bó thon bó chêm Ap chem equilibrium problems
Ap chem equilibrium problems Chem pharma impex
Chem pharma impex Chem connections india
Chem connections india Chem 30 data booklet
Chem 30 data booklet Chem 200
Chem 200 Ap chem equilibrium
Ap chem equilibrium Electrochemistry ap chem
Electrochemistry ap chem Polarity ap chem
Polarity ap chem Dyno chem
Dyno chem Gen chem
Gen chem Chemical equilibrum
Chemical equilibrum