CHEM 3310 Chemical Kinetics Collision Theory Transition State







- Slides: 25

CHEM 3310 Chemical Kinetics Collision Theory & Transition State Theory

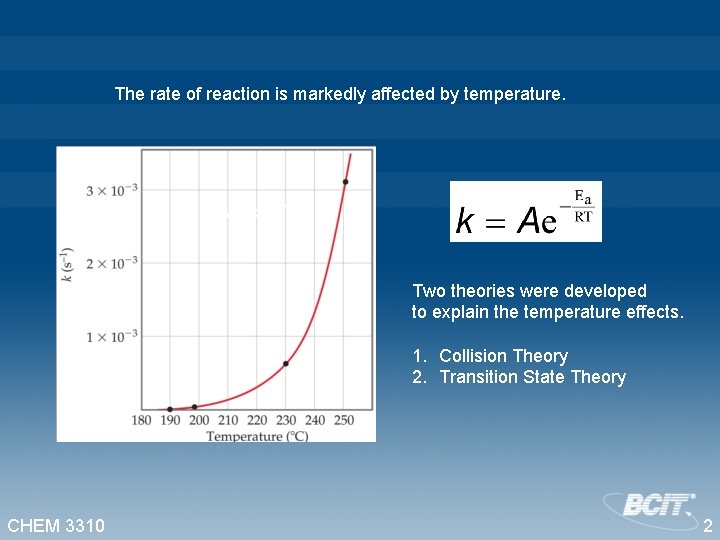
The rate of reaction is markedly affected by temperature. “k versus T” Two theories were developed to explain the temperature effects. 1. Collision Theory 2. Transition State Theory CHEM 3310 2

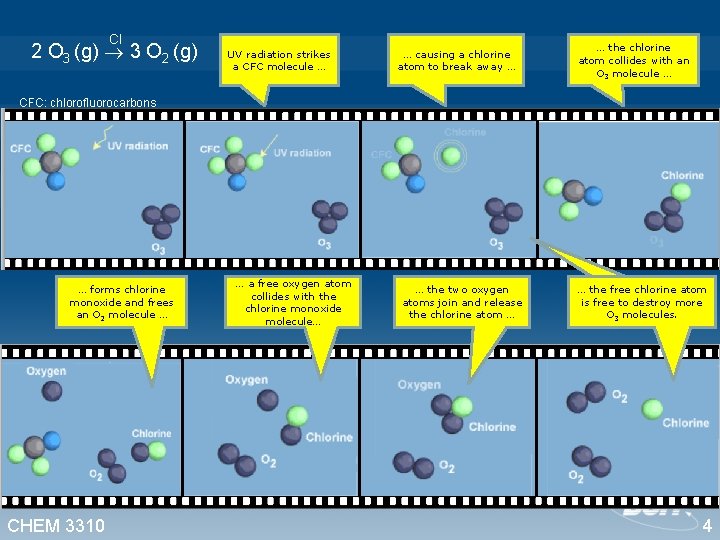
Take the destruction of ozone in the stratosphere. Cl 2 O 3 (g) 3 O 2 (g) Watch animation (http: //www. ucar. edu/learn/images/o 3 split. gif) What is happening at the molecular level? Go back to the basics! CHEM 3310 3

Cl 2 O 3 (g) 3 O 2 (g) UV radiation strikes a CFC molecule … … causing a chlorine atom to break away … … the chlorine atom collides with an O 3 molecule … CFC: chlorofluorocarbons … forms chlorine monoxide and frees an O 2 molecule … CHEM 3310 … a free oxygen atom collides with the chlorine monoxide molecule… … the two oxygen atoms join and release the chlorine atom … … the free chlorine atom is free to destroy more O 3 molecules. 4

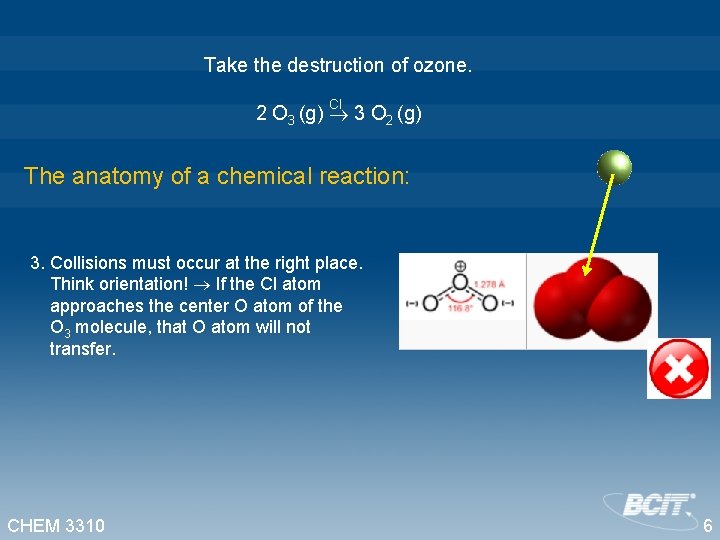
Take the destruction of ozone. Cl 2 O 3 (g) 3 O 2 (g) The anatomy of a chemical reaction: 1. Molecules must interact. Think collisions! Collisions must occur. 2. The more molecules in a confined space, the more likely collisions will occur. Think probability! Rate is proportional to concentrations of the reactants. When O 3 and Cl collide and react, the rate of the reaction will be proportional to the product of the [O 3 ] and [Cl]. CHEM 3310 5

Take the destruction of ozone. Cl 2 O 3 (g) 3 O 2 (g) The anatomy of a chemical reaction: 3. Collisions must occur at the right place. Think orientation! If the Cl atom approaches the center O atom of the O 3 molecule, that O atom will not transfer. CHEM 3310 6

Take the destruction of ozone. Cl 2 O 3 (g) 3 O 2 (g) The anatomy of a chemical reaction: 4. Bonds are broken and new bonds are formed. Think energy! At the time of collision, bonds are stretched and broken as new bonds are made. Breaking these bonds and rearranging the atoms during the collision requires the input of energy. This takes us to the Collision Theory & Transition State Theory. CHEM 3310 7

Collision Theory is based on three postulates: 1. Chemical reactions in the gas phase are due to the collision of the reactant particles. 2. A collision only results in a reaction if a certain threshold energy is exceeded. 3. A collision only results in a reaction if the colliding particles are correctly oriented to one another. We will expand on these postulates in the following slides. CHEM 3310 8

Collision Theory 1. Chemical reactions result from collisions of the reactant particles. This makes sense! CHEM 3310 9

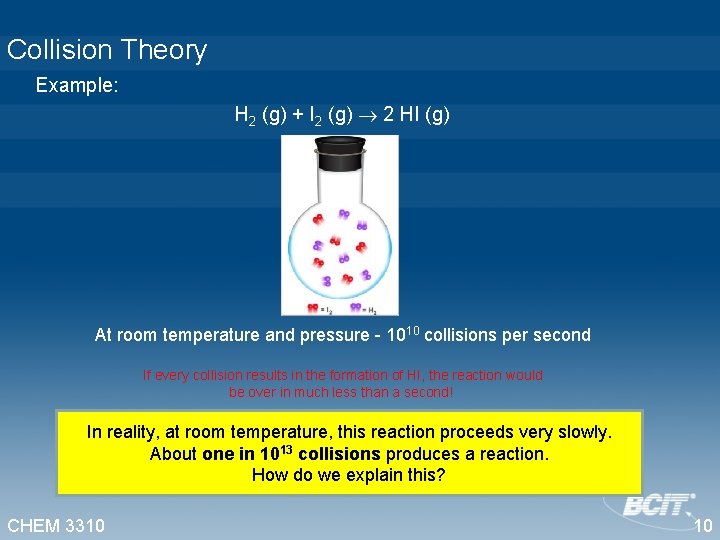
Collision Theory Example: H 2 (g) + I 2 (g) 2 HI (g) At room temperature and pressure - 1010 collisions per second If every collision results in the formation of HI, the reaction would be over in much less than a second! In reality, at room temperature, this reaction proceeds very slowly. About one in 1013 collisions produces a reaction. How do we explain this? CHEM 3310 10

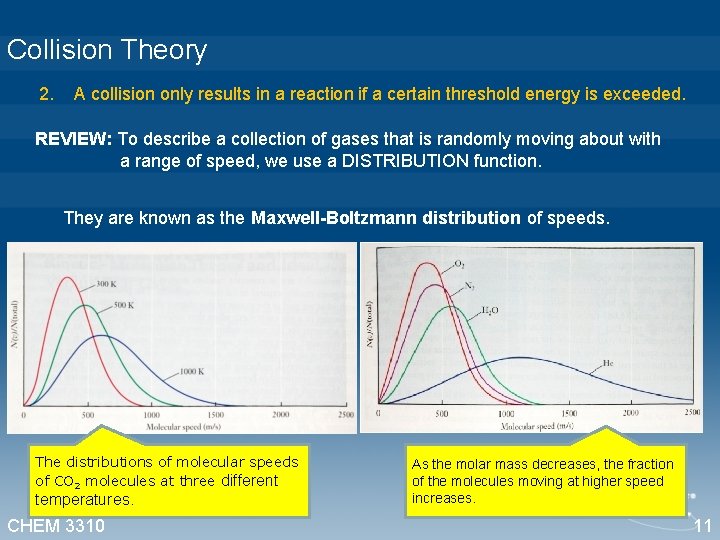
Collision Theory 2. A collision only results in a reaction if a certain threshold energy is exceeded. REVIEW: To describe a collection of gases that is randomly moving about with a range of speed, we use a DISTRIBUTION function. They are known as the Maxwell-Boltzmann distribution of speeds. The distributions of molecular speeds of CO 2 molecules at three different temperatures. CHEM 3310 As the molar mass decreases, the fraction of the molecules moving at higher speed increases. 11

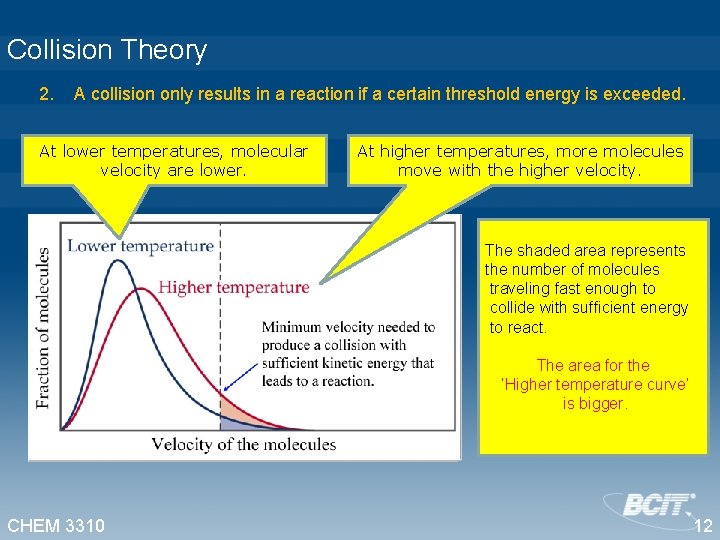
Collision Theory 2. A collision only results in a reaction if a certain threshold energy is exceeded. At lower temperatures, molecular velocity are lower. At higher temperatures, more molecules move with the higher velocity. The shaded area represents the number of molecules traveling fast enough to collide with sufficient energy to react. The area for the ‘Higher temperature curve’ is bigger. CHEM 3310 12

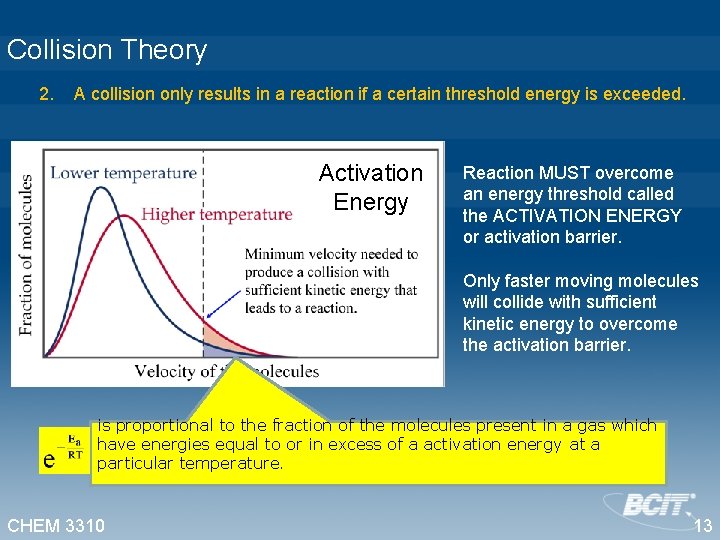
Collision Theory 2. A collision only results in a reaction if a certain threshold energy is exceeded. Activation Energy Reaction MUST overcome an energy threshold called the ACTIVATION ENERGY or activation barrier. Only faster moving molecules will collide with sufficient kinetic energy to overcome the activation barrier. is proportional to the fraction of the molecules present in a gas which have energies equal to or in excess of a activation energy at a particular temperature. CHEM 3310 13

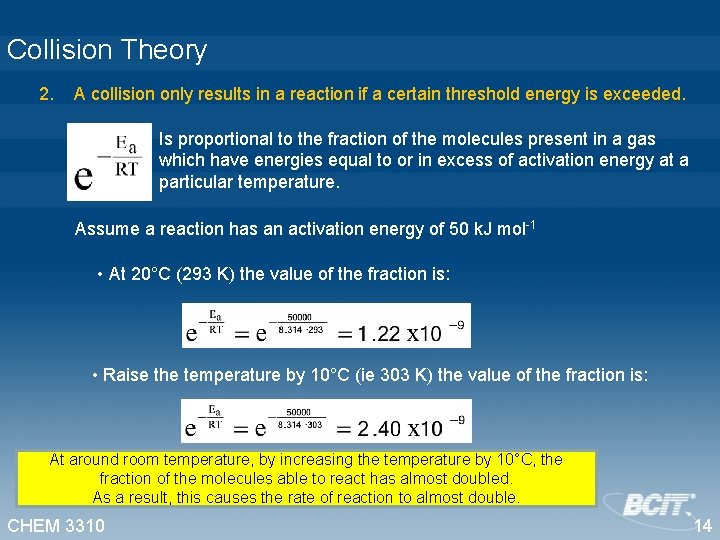
Collision Theory 2. A collision only results in a reaction if a certain threshold energy is exceeded. Is proportional to the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature. Assume a reaction has an activation energy of 50 k. J mol-1 • At 20°C (293 K) the value of the fraction is: • Raise the temperature by 10°C (ie 303 K) the value of the fraction is: At around room temperature, by increasing the temperature by 10°C, the fraction of the molecules able to react has almost doubled. As a result, this causes the rate of reaction to almost double. CHEM 3310 14

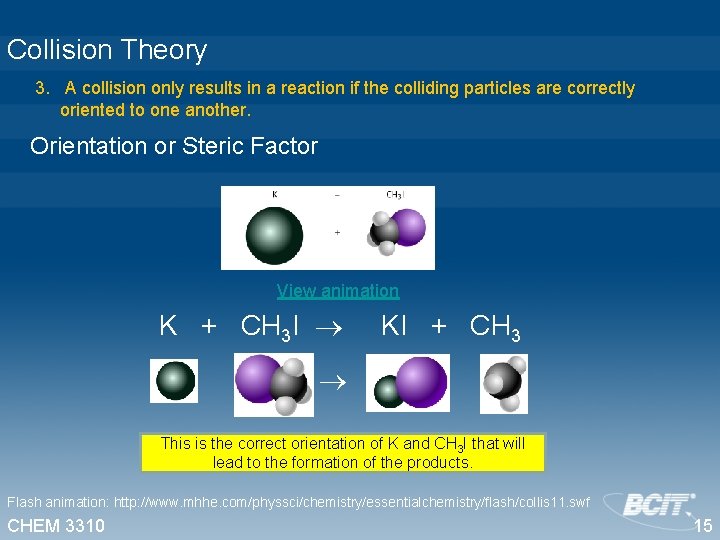
Collision Theory 3. A collision only results in a reaction if the colliding particles are correctly oriented to one another. Orientation or Steric Factor View animation K + CH 3 I KI + CH 3 This is the correct orientation of K and CH 3 I that will lead to the formation of the products. Flash animation: http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/collis 11. swf CHEM 3310 15

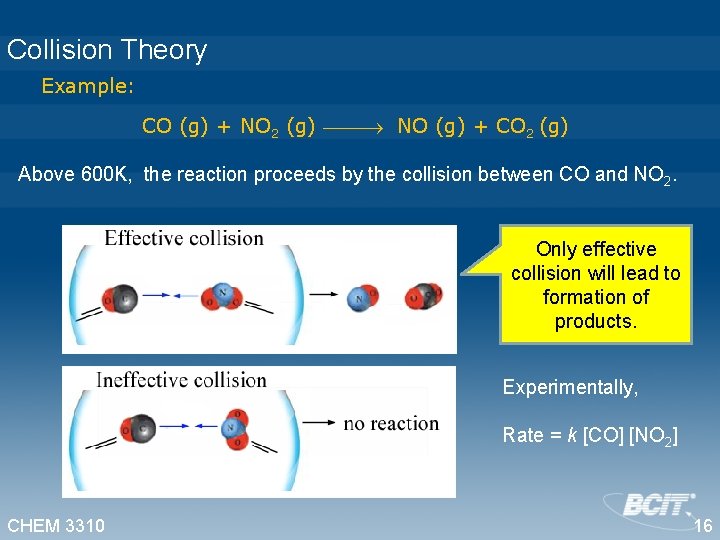
Collision Theory Example: CO (g) + NO 2 (g) NO (g) + CO 2 (g) Above 600 K, the reaction proceeds by the collision between CO and NO 2. Only effective collision will lead to formation of products. Experimentally, Rate = k [CO] [NO 2] CHEM 3310 16

3. A collision only results in a reaction if the colliding particles are correctly oriented to one another. Frequency Factor, A, in Arrhenius Equation • A is a constant that indicates how many collisions have the correct orientation that could lead to the formation of products. • The units of the A are identical to those of the rate constant. (eg – For first order reaction, A has the units of s− 1, is of the order of 1013 s-1) CHEM 3310 17

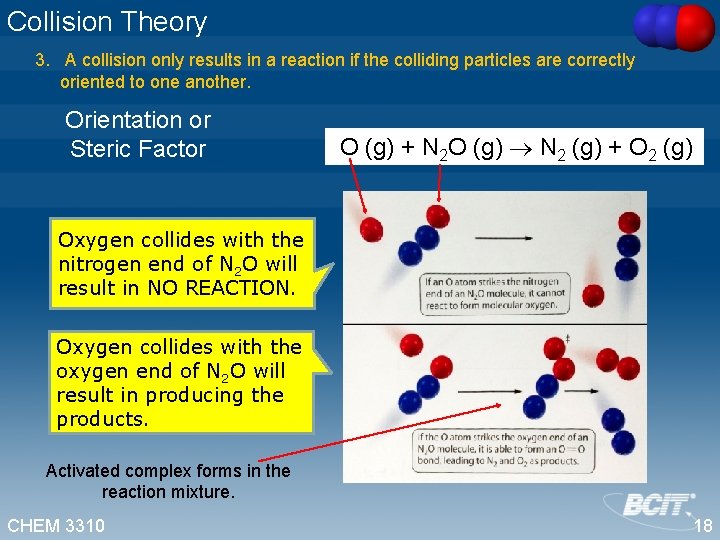
Collision Theory 3. A collision only results in a reaction if the colliding particles are correctly oriented to one another. Orientation or Steric Factor O (g) + N 2 O (g) N 2 (g) + O 2 (g) Oxygen collides with the nitrogen end of N 2 O will result in NO REACTION. Oxygen collides with the oxygen end of N 2 O will result in producing the products. Activated complex forms in the reaction mixture. CHEM 3310 18

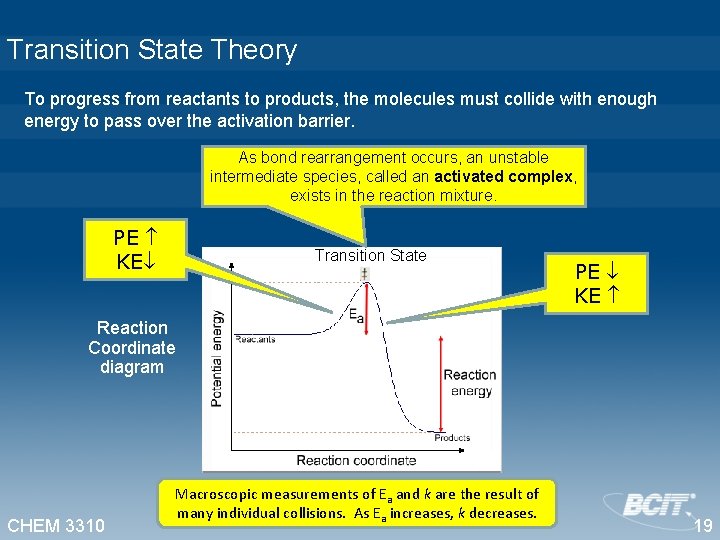
Transition State Theory To progress from reactants to products, the molecules must collide with enough energy to pass over the activation barrier. As bond rearrangement occurs, an unstable intermediate species, called an activated complex, exists in the reaction mixture. PE KE Transition State PE KE Reaction Coordinate diagram CHEM 3310 Macroscopic measurements of Ea and k are the result of many individual collisions. As Ea increases, k decreases. 19

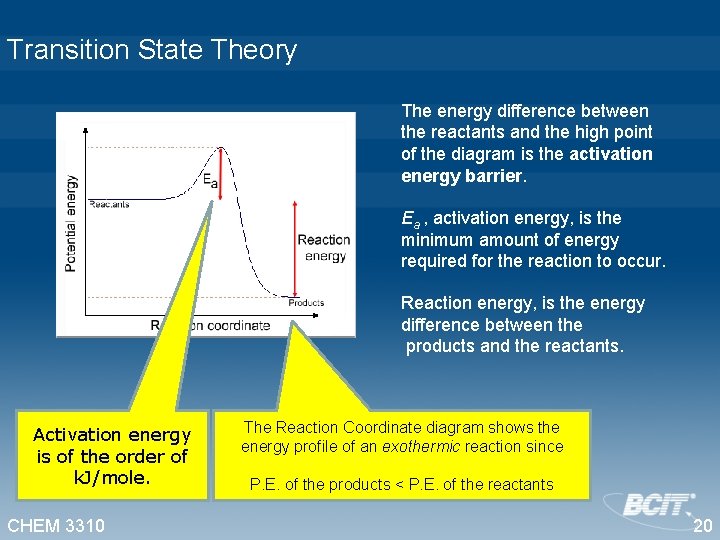
Transition State Theory The energy difference between the reactants and the high point of the diagram is the activation energy barrier. Ea , activation energy, is the minimum amount of energy required for the reaction to occur. Reaction energy, is the energy difference between the products and the reactants. Activation energy is of the order of k. J/mole. CHEM 3310 The Reaction Coordinate diagram shows the energy profile of an exothermic reaction since P. E. of the products < P. E. of the reactants 20

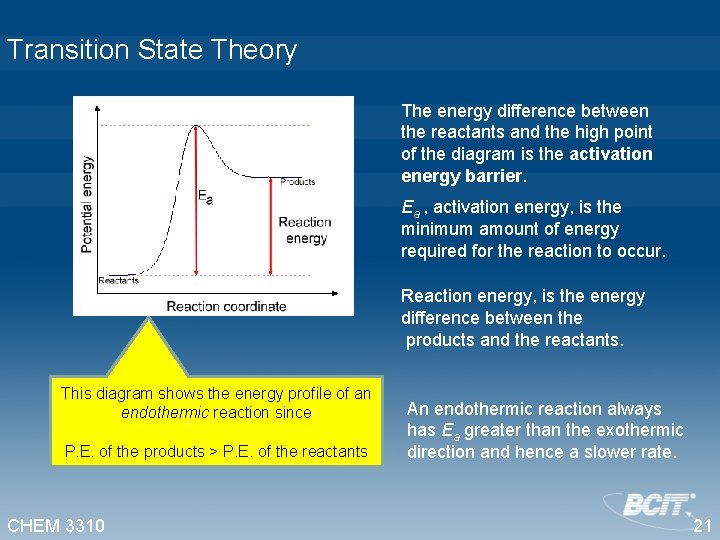
Transition State Theory The energy difference between the reactants and the high point of the diagram is the activation energy barrier. Ea , activation energy, is the minimum amount of energy required for the reaction to occur. Reaction energy, is the energy difference between the products and the reactants. This diagram shows the energy profile of an endothermic reaction since P. E. of the products > P. E. of the reactants CHEM 3310 An endothermic reaction always has Ea greater than the exothermic direction and hence a slower rate. 21

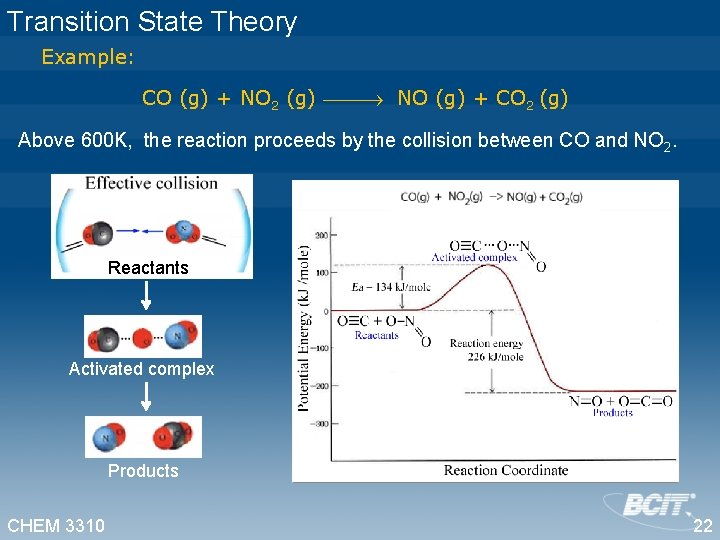
Transition State Theory Example: CO (g) + NO 2 (g) NO (g) + CO 2 (g) Above 600 K, the reaction proceeds by the collision between CO and NO 2. Reactants Activated complex Products CHEM 3310 22

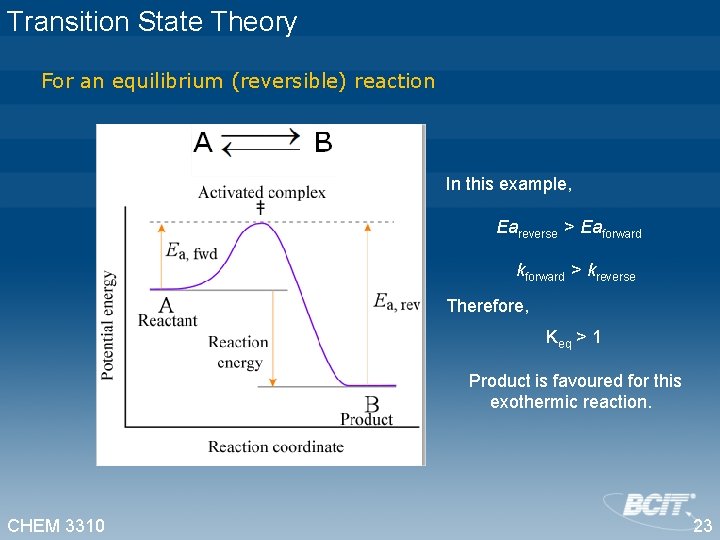
Transition State Theory For an equilibrium (reversible) reaction In this example, Eareverse > Eaforward kforward > kreverse Therefore, Keq > 1 Product is favoured for this exothermic reaction. CHEM 3310 23

Summary The Collision Theory • Assumes a collision between reactants needs to happen before a reaction can take place. • the majority of collisions do not lead to a reaction, but only those in which the colliding species have: • A kinetic energy greater than a certain minimum called the activation energy, Ea • The correct spacial orientation with respect to each other. CHEM 3310 The Transition State Theory • During a reaction, an increase in potential energy corresponds to an energy barrier over which the reactant molecules must pass if the reaction is to proceed. • The transition state occurs at the maximum of this energy barrier. • The energy difference between the reactants and the potential energy maximum is referred to as the activation energy. • The energy difference between the potential energy of the products and reactants is referred to as the reaction energy. 24

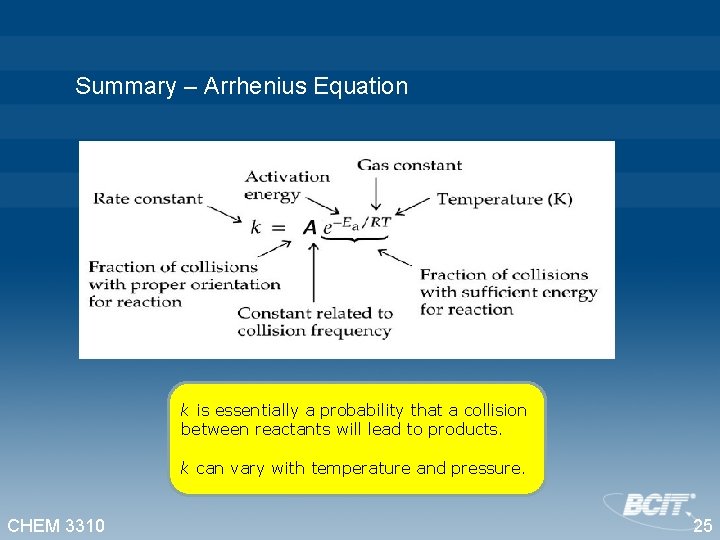
Summary – Arrhenius Equation k is essentially a probability that a collision between reactants will lead to products. k can vary with temperature and pressure. CHEM 3310 25