CHEM 3310 Chemical Kinetics Catalysts Catalysts A catalyst


- Slides: 15

CHEM 3310 Chemical Kinetics Catalysts

Catalysts A catalyst is a substance that increases the rate of the reaction but is neither created nor destroyed in the process. Catalysts can be divided into two broad categories. Homogeneous catalysts are those that are in the same phase as the reacting substances. Heterogeneous catalysts are in a different phase from the reacting species. CHEM 3310 2

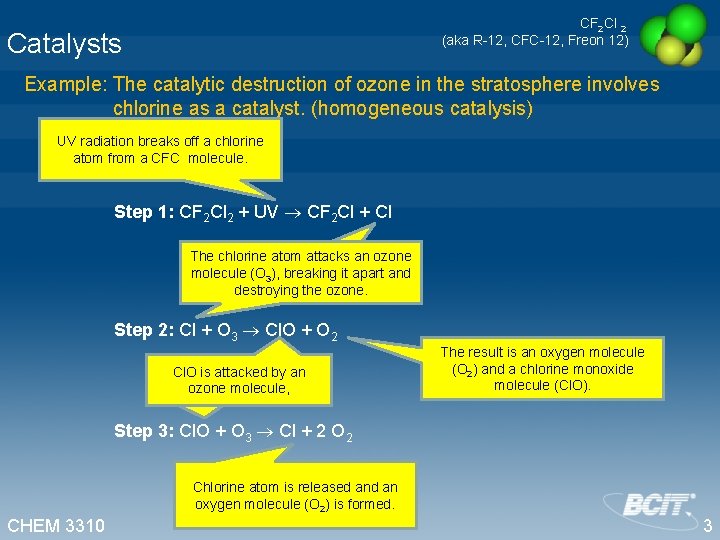
CF 2 Cl 2 (aka R-12, CFC-12, Freon 12) Catalysts Example: The catalytic destruction of ozone in the stratosphere involves chlorine as a catalyst. (homogeneous catalysis) UV radiation breaks off a chlorine atom from a CFC molecule. Step 1: CF 2 Cl 2 + UV CF 2 Cl + Cl The chlorine atom attacks an ozone molecule (O 3), breaking it apart and destroying the ozone. Step 2: Cl + O 3 Cl. O + O 2 Cl. O is attacked by an ozone molecule, The result is an oxygen molecule (O 2) and a chlorine monoxide molecule (Cl. O). Step 3: Cl. O + O 3 Cl + 2 O 2 Chlorine atom is released an oxygen molecule (O 2) is formed. CHEM 3310 3

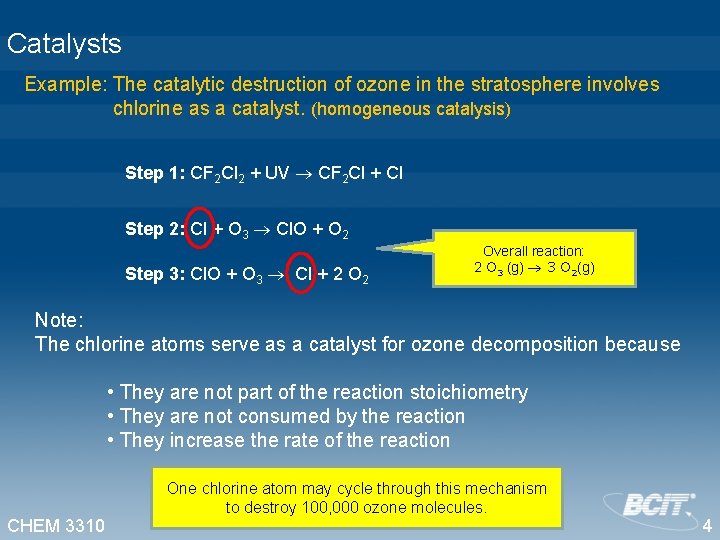
Catalysts Example: The catalytic destruction of ozone in the stratosphere involves chlorine as a catalyst. (homogeneous catalysis) Step 1: CF 2 Cl 2 + UV CF 2 Cl + Cl Step 2: Cl + O 3 Cl. O + O 2 Step 3: Cl. O + O 3 Cl + 2 Overall reaction: 2 O 3 (g) 3 O 2(g) Note: The chlorine atoms serve as a catalyst for ozone decomposition because • They are not part of the reaction stoichiometry • They are not consumed by the reaction • They increase the rate of the reaction CHEM 3310 One chlorine atom may cycle through this mechanism to destroy 100, 000 ozone molecules. 4

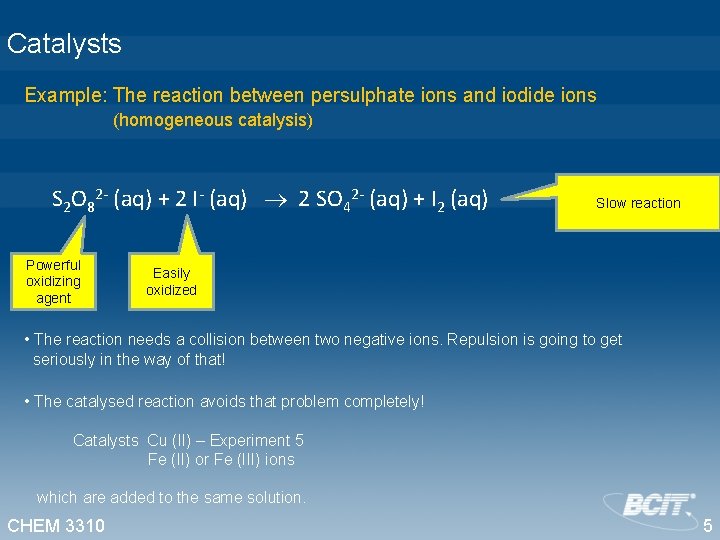
Catalysts Example: The reaction between persulphate ions and iodide ions (homogeneous catalysis) S 2 O 82 - (aq) + 2 I- (aq) 2 SO 42 - (aq) + I 2 (aq) Powerful oxidizing agent Slow reaction Easily oxidized • The reaction needs a collision between two negative ions. Repulsion is going to get seriously in the way of that! • The catalysed reaction avoids that problem completely! Catalysts Cu (II) – Experiment 5 Fe (II) or Fe (III) ions which are added to the same solution. CHEM 3310 5

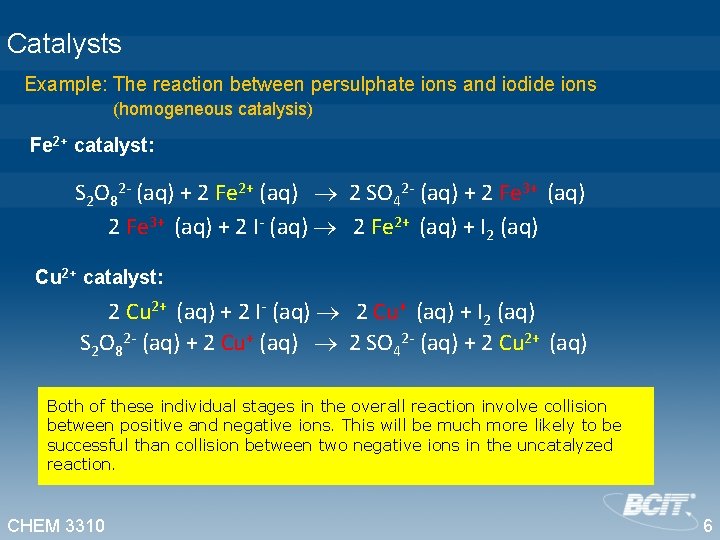
Catalysts Example: The reaction between persulphate ions and iodide ions (homogeneous catalysis) Fe 2+ catalyst: S 2 O 82 - (aq) + 2 Fe 2+ (aq) 2 SO 42 - (aq) + 2 Fe 3+ (aq) + 2 I- (aq) 2 Fe 2+ (aq) + I 2 (aq) Cu 2+ catalyst: 2 Cu 2+ (aq) + 2 I- (aq) 2 Cu+ (aq) + I 2 (aq) S 2 O 82 - (aq) + 2 Cu+ (aq) 2 SO 42 - (aq) + 2 Cu 2+ (aq) Both of these individual stages in the overall reaction involve collision between positive and negative ions. This will be much more likely to be successful than collision between two negative ions in the uncatalyzed reaction. CHEM 3310 6

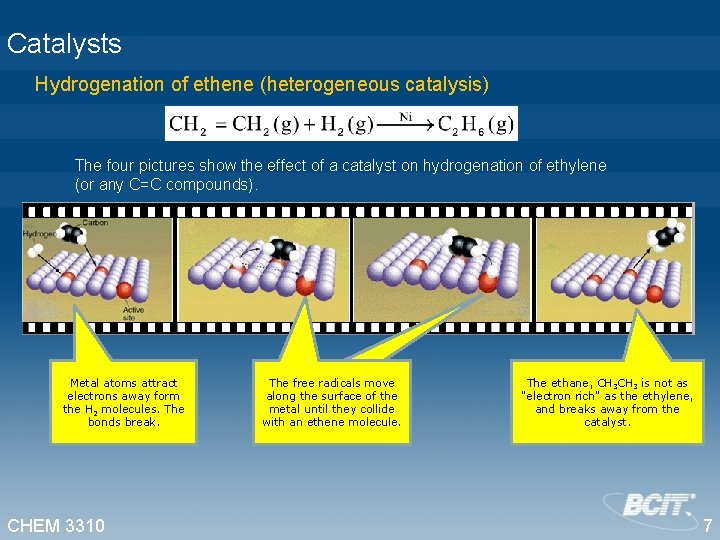
Catalysts Hydrogenation of ethene (heterogeneous catalysis) The four pictures show the effect of a catalyst on hydrogenation of ethylene (or any C=C compounds). Metal atoms attract electrons away form the H 2 molecules. The bonds break. CHEM 3310 The free radicals move along the surface of the metal until they collide with an ethene molecule. The ethane, CH 3 is not as "electron rich" as the ethylene, and breaks away from the catalyst. 7

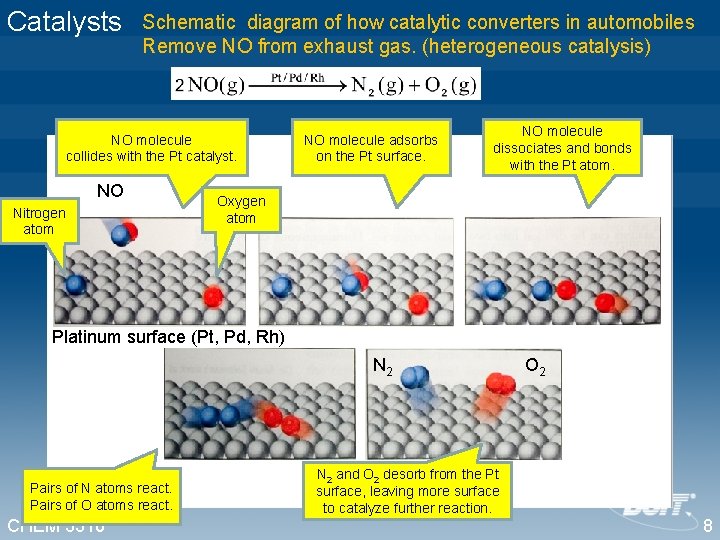
Catalysts Schematic diagram of how catalytic converters in automobiles Remove NO from exhaust gas. (heterogeneous catalysis) NO molecule collides with the Pt catalyst. NO Nitrogen atom NO molecule adsorbs on the Pt surface. NO molecule dissociates and bonds with the Pt atom. Oxygen atom Platinum surface (Pt, Pd, Rh) N 2 Pairs of N atoms react. Pairs of O atoms react. CHEM 3310 O 2 N 2 and O 2 desorb from the Pt surface, leaving more surface to catalyze further reaction. 8

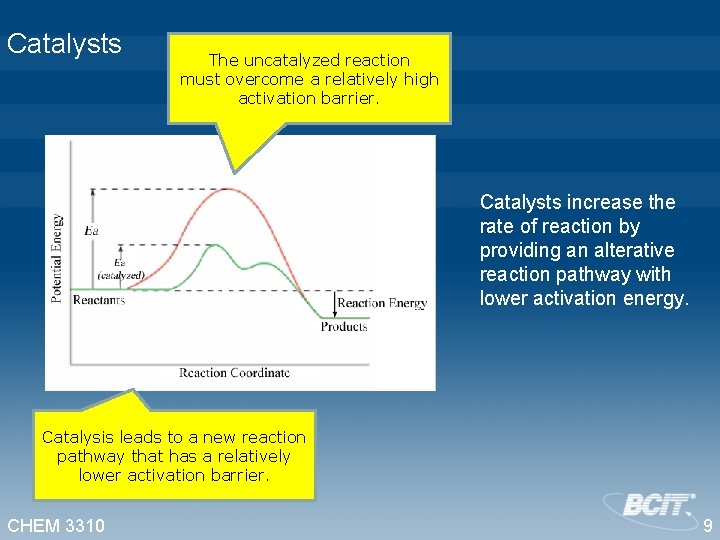
Catalysts The uncatalyzed reaction must overcome a relatively high activation barrier. Catalysts increase the rate of reaction by providing an alterative reaction pathway with lower activation energy. Catalysis leads to a new reaction pathway that has a relatively lower activation barrier. CHEM 3310 9

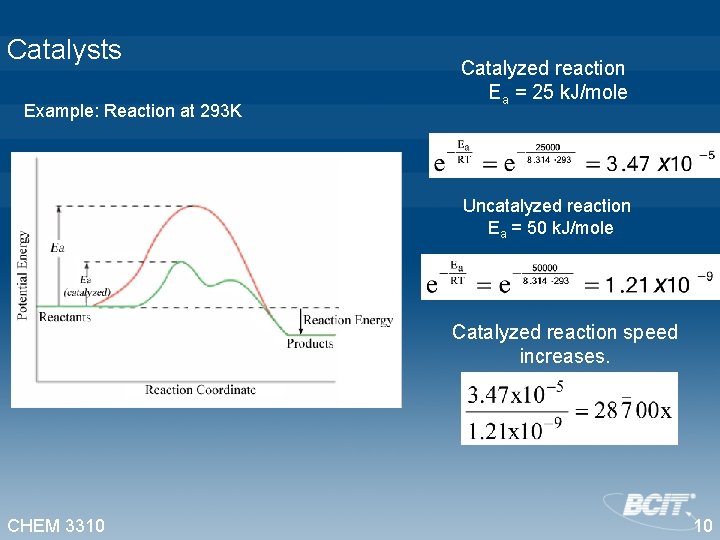
Catalysts Example: Reaction at 293 K Catalyzed reaction Ea = 25 k. J/mole Uncatalyzed reaction Ea = 50 k. J/mole Catalyzed reaction speed increases. CHEM 3310 10

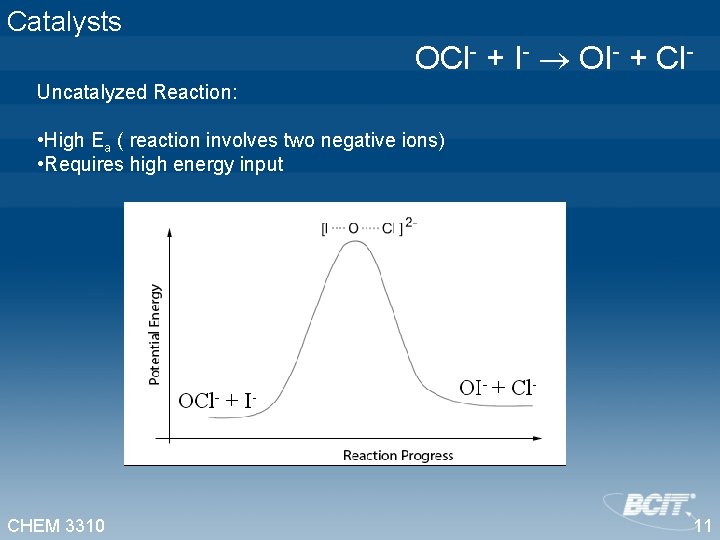
Catalysts OCl- + I- OI- + Cl. Uncatalyzed Reaction: • High Ea ( reaction involves two negative ions) • Requires high energy input CHEM 3310 11

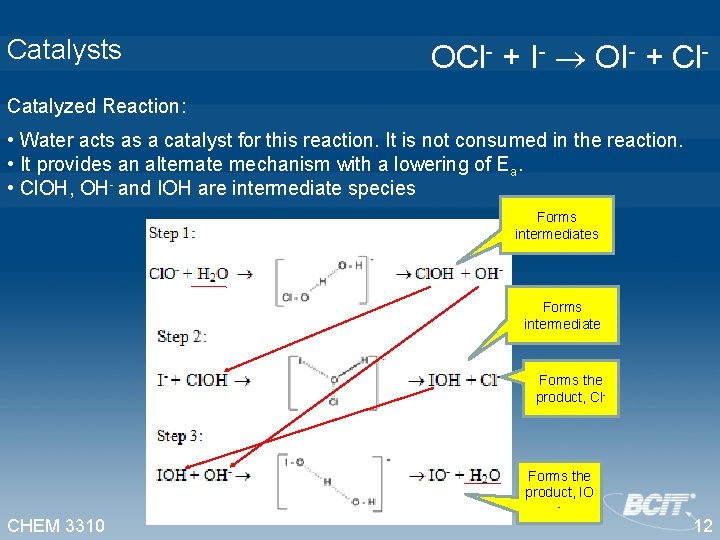
Catalysts OCl- + I- OI- + Cl- Catalyzed Reaction: • Water acts as a catalyst for this reaction. It is not consumed in the reaction. • It provides an alternate mechanism with a lowering of Ea. • Cl. OH, OH- and IOH are intermediate species Forms intermediate Forms the product, Cl- CHEM 3310 Forms the product, IO - 12

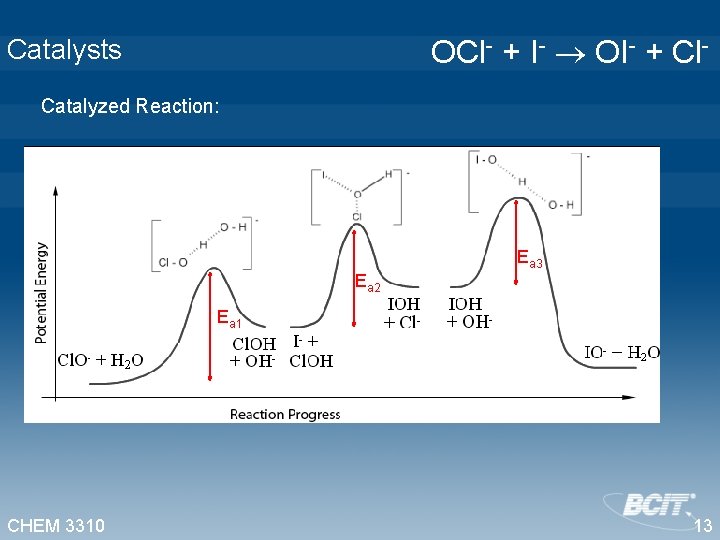
Catalysts OCl- + I- OI- + Cl- Catalyzed Reaction: Ea 3 Ea 2 Ea 1 CHEM 3310 13

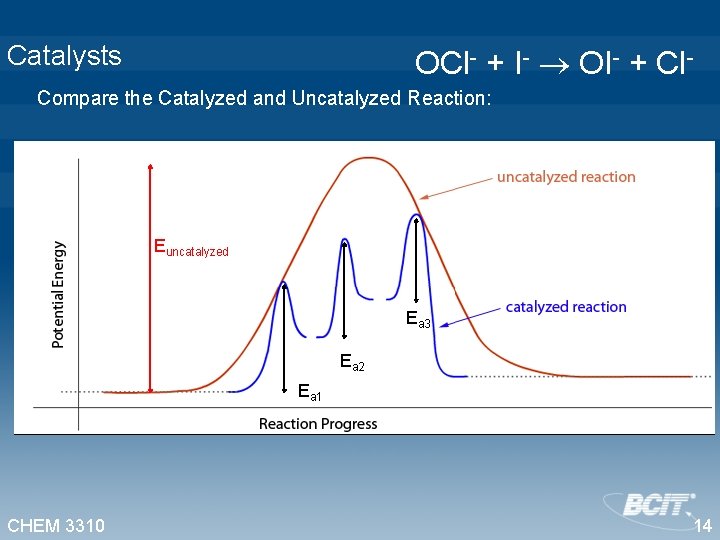
Catalysts OCl- + I- OI- + Cl- Compare the Catalyzed and Uncatalyzed Reaction: Euncatalyzed Ea 3 Ea 2 Ea 1 CHEM 3310 14

Catalysts • Important in billion dollar industries such as petroleum refining • Research of materials and design process for better catalysts • Selective so that it speeds up one reaction and not all the reactions • Historically, selection of catalysts is by trial and errors Modern days, catalysts are better understood by: 1. Reaction mechanisms 2. Molecular structure The importance of 3. Material properties Chemistry!! CHEM 3310 15