Chem 231 128 Lecture Introduction Goals of Course














- Slides: 23

Chem. 231 – 1/28 Lecture

Introduction Goals of Course • Discussion of More Practical Aspects of Separation Science • Provide Specific Lab Training in Use of Chromatography Instruments and Simple Separations (e. g. Extractions) • Facilitate Semi-Independent Student Learning • Improve Communication Skills

Syllabus Class/Discussion Meeting Times • Not exactly as in syllabus • We typically have extended discussions and instrument demonstrations in the beginning • We will end discussion “lectures” about half the way through the semester; Discussions will then occur for 3 rd lab oral presentations • Some work can be performed outside of class hours (once first lab is completed) provided work doesn’t interfere with other classes and follows department policy

Syllabus Website + Discussion • Additional Course Information on Website • Discussion Topics – In past, I covered “practical” topics I thought were important – I expect topics to be divided between general “practical” information and information specific for the experiments to be performed

Syllabus Laboratory Experiments • Set 1 Labs – Relatively simple experiments to demonstrate use of HPLC and GC – Will need to do both laboratory experiments – One will include quantitative work that will be reported while the other will just be for competency

Syllabus Laboratory Experiments • Set 2 Labs – Both labs use extraction methods as would be typical for extraction of compounds from complicated matrices – HPLC lab will use solid phase extraction to trap phenols from an aqueous matrix – GC lab will use solid phase microextraction to trap volatile compounds from gas

Syllabus Laboratory Experiments • Set 3 Labs – The focus of these experiments will be to derivatize fatty acids and carbonyl compounds for subsequent analysis by GC and HPLC – Students will provide additional samples of interest

Syllabus Laboratory Experiments • Term Project Labs – These are meant to be more independent – Students can isolate an ingredient from a household product or – Students can work on a separation using equipment beyond that used in the first 3 experiments (e. g. use HPLC with fluorescence detection)

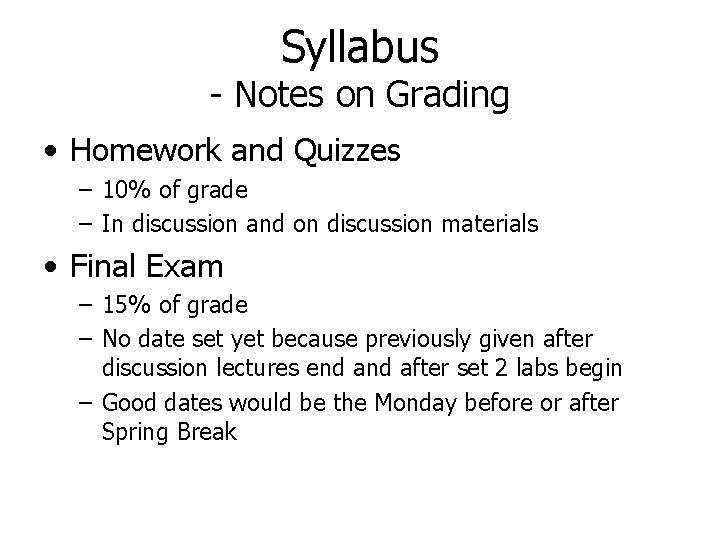
Syllabus - Notes on Grading • Homework and Quizzes – 10% of grade – In discussion and on discussion materials • Final Exam – 15% of grade – No date set yet because previously given after discussion lectures end after set 2 labs begin – Good dates would be the Monday before or after Spring Break

Syllabus - Notes on Grading (2) • Lab Reports – 75% of grade – Will submit 4 formal written reports (one for each lab + for the term project) (65% of grade) – Will submit one ungraded report for the other set 1 lab (e. g. formal for HPLC and informal for GC) – Will give one oral report on your particular sample in the (Set 3) derivatization lab (10% of grade)

Lab Safety • Safety Equipment Required – Goggles, gloves and lab coats needed – Must wear long pants and shoes covering feet below ankle – Goggles and lab coats should be worn while running instruments, but gloves only needed when handling chemicals – Gloves should not be worn outside of labs (people get concerned that you are spreading toxic compounds) – Gloves can both protect your hands from chemicals (e. g. from dinitrophenylhydrozene) and protect your samples from your hands (e. g. from fatty acids)

Other Lab Equipment Needed • Lab Notebook (and pen) • Lab Instructions (see e. g. Set 1 Lab Instructions) • Notes from Discussion • Memory stick/floppy drive

Discussion Topics • Practical Aspects of Separation Science – example: demonstration and discussion of how SPE cartridges work and are used • Specific Tools Needed for Our Labs – example: how to set up file folders to save data using the Agilent HPLC

Today’s Discussion Topic: Data Processing • • How to Set Up Folders Saving Raw and Processed Data Excel Processing of Raw Data External Standard Calibration Using Excel

Today in Lab • Check in to lockers

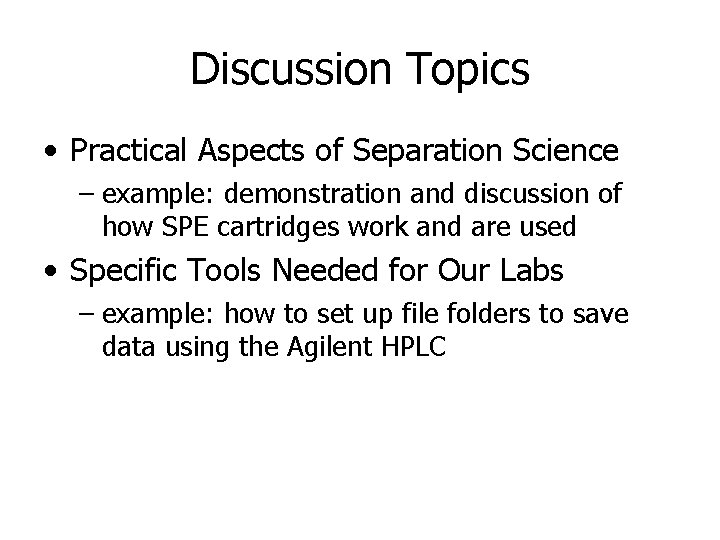
Data Processing How to Set Up Folders • Whenever possible, use your own folders • Advantages: – All your stuff should go there – Nobody else’s stuff should go there – Easier to find data/methods and less likely to have data overwritten • Disadvantages: – it takes a little time to set up Example: Chemstation

Data Processing Saving Raw Data I • Raw Chromatographic data consists of time and response(s) (response for a single channel instrument to responses for multichannel instruments) • Most software programs allow you to obtain raw data • For some instruments (Buck GC), data presented using Excel looks nicer than software chromatograms (with the exception of no integration)

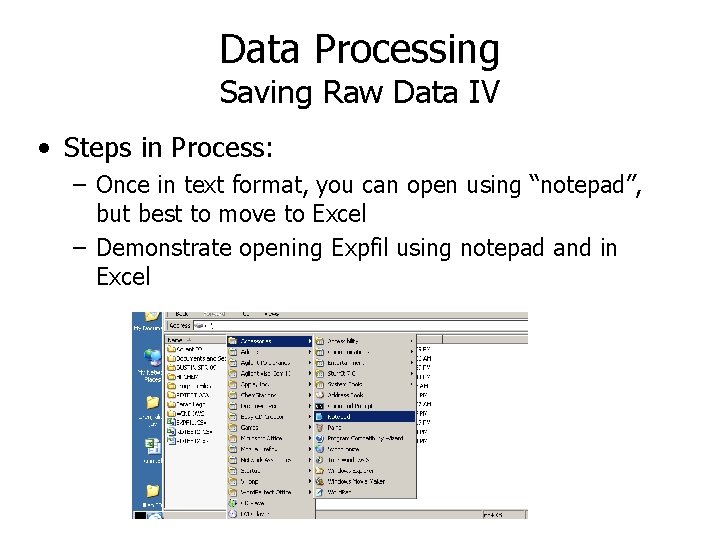
Data Processing Saving Raw Data II • Steps in Process: – Open file (in Chem. Station, you need to be in Data Analysis mode) Example: Chemstation

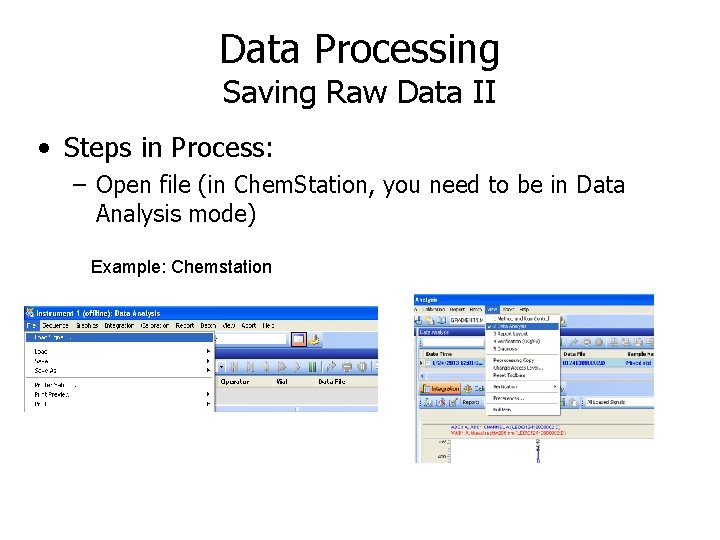
Data Processing Saving Raw Data III • Steps in Process: – Look for way to “export” file or “save file as” with the output being a “text” file (these usually have a. txt extension) In Chem. Station, text file made as “CSV” file

Data Processing Saving Raw Data IV • Steps in Process: – Once in text format, you can open using “notepad”, but best to move to Excel – Demonstrate opening Expfil using notepad and in Excel

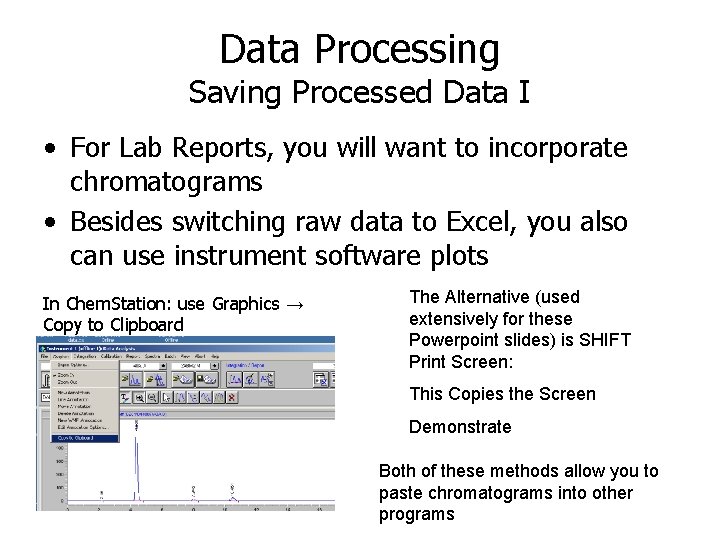
Data Processing Saving Processed Data I • For Lab Reports, you will want to incorporate chromatograms • Besides switching raw data to Excel, you also can use instrument software plots In Chem. Station: use Graphics → Copy to Clipboard The Alternative (used extensively for these Powerpoint slides) is SHIFT Print Screen: This Copies the Screen Demonstrate Both of these methods allow you to paste chromatograms into other programs

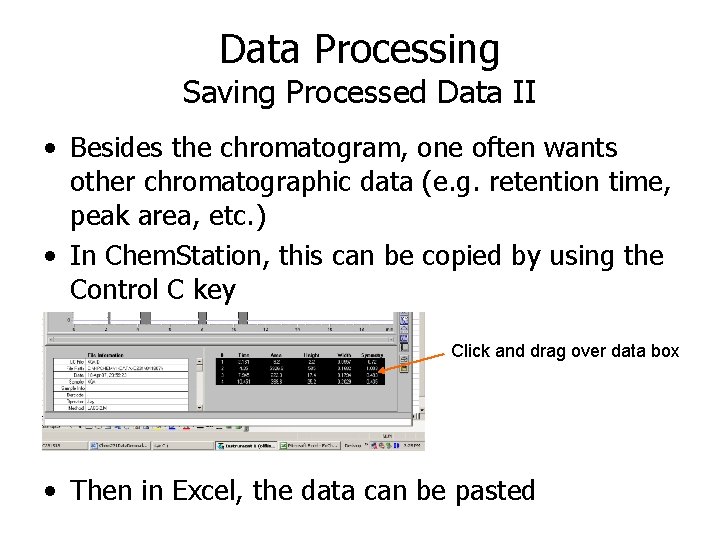
Data Processing Saving Processed Data II • Besides the chromatogram, one often wants other chromatographic data (e. g. retention time, peak area, etc. ) • In Chem. Station, this can be copied by using the Control C key Click and drag over data box • Then in Excel, the data can be pasted

Data Processing Data Smoothing in Excel • Demonstrate using trendline for a running average and also using average function with Excel
 Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Meth eth prop but
Meth eth prop but 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad General goals and specific goals
General goals and specific goals Examples of generic goals and product-specific goals
Examples of generic goals and product-specific goals 13 malaysian patient safety goals
13 malaysian patient safety goals Acf 231
Acf 231 Article 231 of the treaty of versailles
Article 231 of the treaty of versailles 132 213
132 213 Phy 231 msu
Phy 231 msu Decreto 231 del 2007
Decreto 231 del 2007 Hino 231 letra
Hino 231 letra Gezang 231
Gezang 231 Application of shift register
Application of shift register Cs 231
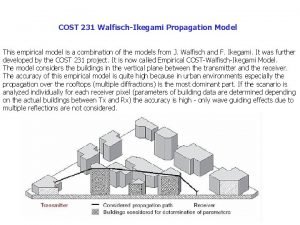
Cs 231 Walfisch ikegami model
Walfisch ikegami model Draw 231 with base ten blocks
Draw 231 with base ten blocks 040 231 3666
040 231 3666 Mvcnn pytorch
Mvcnn pytorch Standford cs231
Standford cs231 Bbm 231
Bbm 231 Maksud 231
Maksud 231 German territorial losses
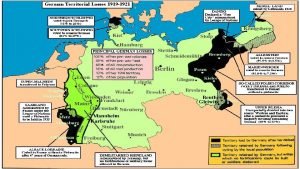
German territorial losses