CHE 333 Class 8 Precipitation Hardening Precipitation Hardening




- Slides: 11

CHE 333 Class 8 Precipitation Hardening

Precipitation Hardening is a NON EQUILIBRIUM heat treatment procedure. Process involves a solution heat treatment, that is transformation to a single phase, followed by a quench, again to suppress a phase transformation. The major difference between age hardening and martensite transformation is that the high temperature phase is retained to low temperatures.

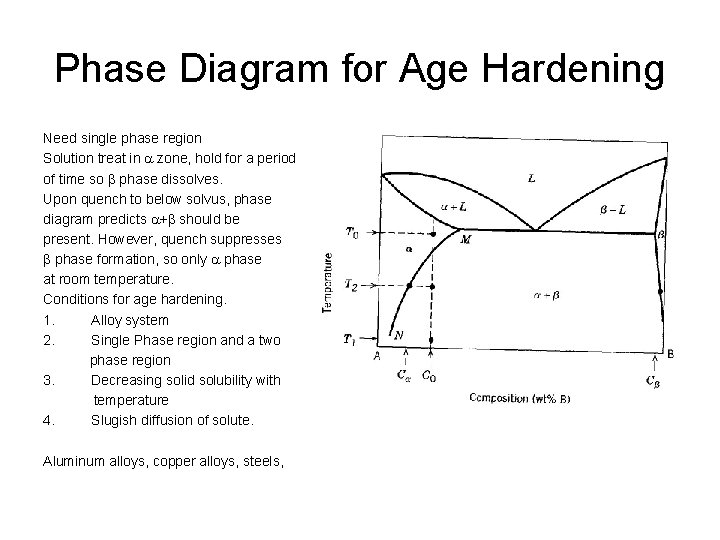
Phase Diagram for Age Hardening Need single phase region Solution treat in a zone, hold for a period of time so b phase dissolves. Upon quench to below solvus, phase diagram predicts a+b should be present. However, quench suppresses b phase formation, so only a phase at room temperature. Conditions for age hardening. 1. Alloy system 2. Single Phase region and a two phase region 3. Decreasing solid solubility with temperature 4. Slugish diffusion of solute. Aluminum alloys, copper alloys, steels,

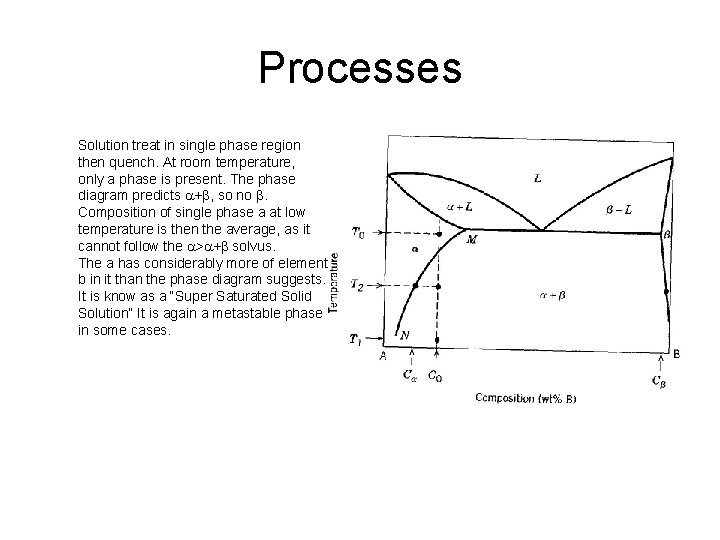
Processes Solution treat in single phase region then quench. At room temperature, only a phase is present. The phase diagram predicts a+b, so no b. Composition of single phase a at low temperature is then the average, as it cannot follow the a>a+b solvus. The a has considerably more of element b in it than the phase diagram suggests. It is know as a “Super Saturated Solid Solution” It is again a metastable phase in some cases.

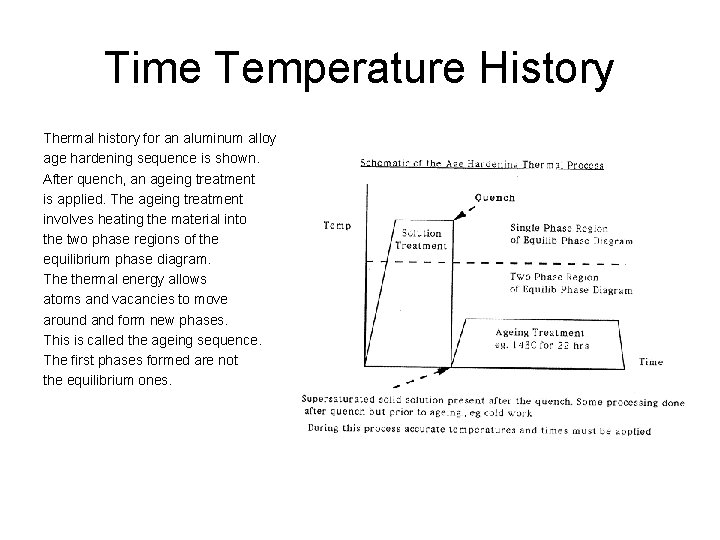
Time Temperature History Thermal history for an aluminum alloy age hardening sequence is shown. After quench, an ageing treatment is applied. The ageing treatment involves heating the material into the two phase regions of the equilibrium phase diagram. The thermal energy allows atoms and vacancies to move around and form new phases. This is called the ageing sequence. The first phases formed are not the equilibrium ones.

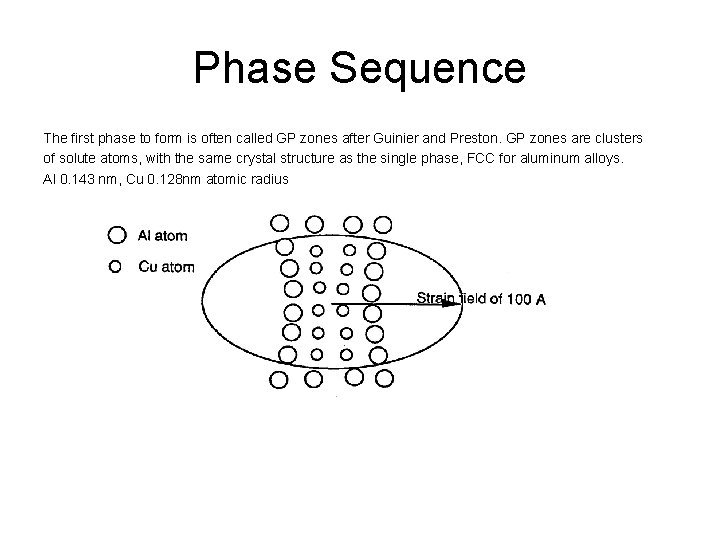
Phase Sequence The first phase to form is often called GP zones after Guinier and Preston. GP zones are clusters of solute atoms, with the same crystal structure as the single phase, FCC for aluminum alloys. Al 0. 143 nm, Cu 0. 128 nm atomic radius

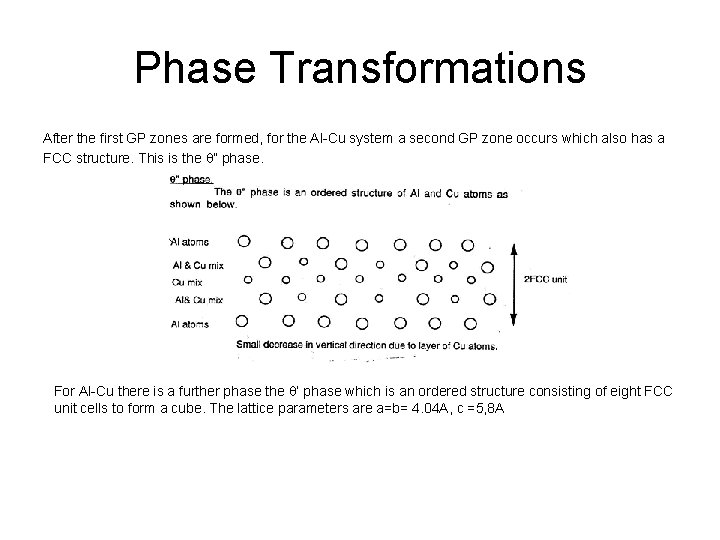
Phase Transformations After the first GP zones are formed, for the Al-Cu system a second GP zone occurs which also has a FCC structure. This is the q” phase. For Al-Cu there is a further phase the q’ phase which is an ordered structure consisting of eight FCC unit cells to form a cube. The lattice parameters are a=b= 4. 04 A, c =5, 8 A

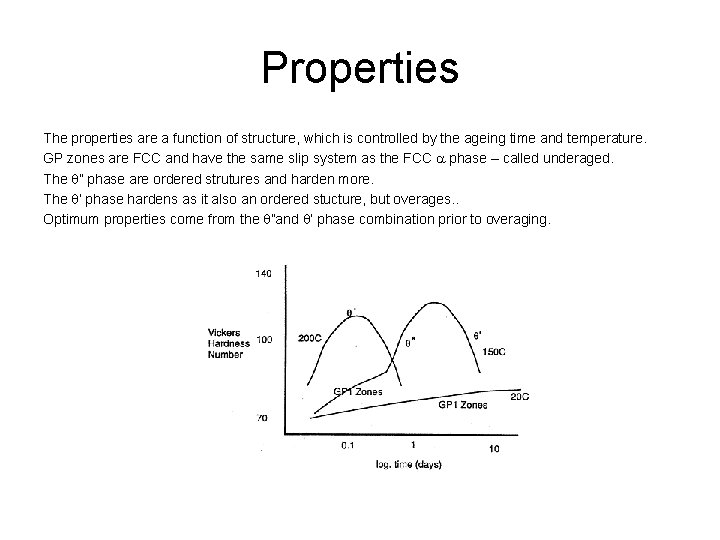
Properties The properties are a function of structure, which is controlled by the ageing time and temperature. GP zones are FCC and have the same slip system as the FCC a phase – called underaged. The q” phase are ordered strutures and harden more. The q’ phase hardens as it also an ordered stucture, but overages. . Optimum properties come from the q”and q’ phase combination prior to overaging.

Structures GP zones in Al-4%Cu, 540 C for 1 hour 130 C for 16 hours 1, 000 mag {100} planes, 100 A diam 10% atomic diameter difference growth as plates. GP zones Al-16%Ag 520 C 160 C for 5 days 200, 000 mag Minimal strain, spheres

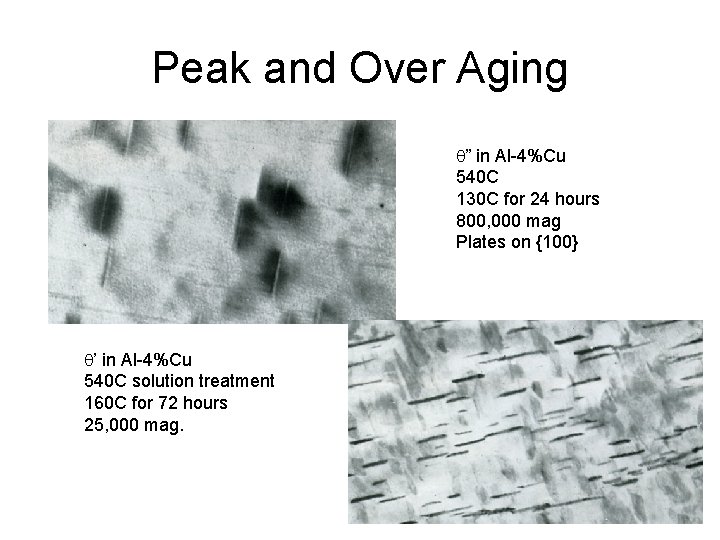
Peak and Over Aging q” in Al-4%Cu 540 C 130 C for 24 hours 800, 000 mag Plates on {100} q’ in Al-4%Cu 540 C solution treatment 160 C for 72 hours 25, 000 mag.

Homework 1. Upon quenching a 0. 4 wt% carbon steel from 950 C to -50 C, what phases will be present and what will be the composition? 1. If an optimum age hardening treatment is 150 C for 22 hours, what is the effect of raising the temperature to 180 C. What is the effect of lengthening the time at 150 C to 5 days or decreasing the time to 12 hours. Indicate the effects by diagrams? 1. What conditions need to be met for a material to be age hardenable?
 Non equilibrium heat treatment
Non equilibrium heat treatment Che 333
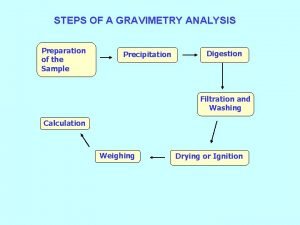
Che 333 Steps of gravimetric analysis
Steps of gravimetric analysis Co precipitation and post precipitation
Co precipitation and post precipitation 5 periodi con proposizioni oggettive
5 periodi con proposizioni oggettive La vita che avrai non sarà mai distante dall'amore che dai
La vita che avrai non sarà mai distante dall'amore che dai Che che kooley
Che che kooley Facesti come quei che va di notte che porta il lume
Facesti come quei che va di notte che porta il lume Binary domain torrent
Binary domain torrent Ece333
Ece333 Komax 341
Komax 341 Ofc<333
Ofc<333