CHE 333 Class 23 Ceramics Ceramic Structures Two


- Slides: 14

CHE 333 Class 23. Ceramics

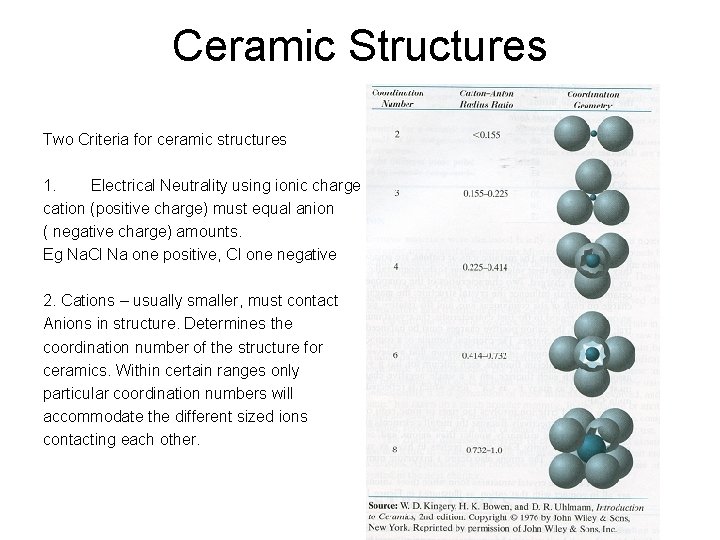
Ceramic Structures Two Criteria for ceramic structures 1. Electrical Neutrality using ionic charge cation (positive charge) must equal anion ( negative charge) amounts. Eg Na. Cl Na one positive, Cl one negative 2. Cations – usually smaller, must contact Anions in structure. Determines the coordination number of the structure for ceramics. Within certain ranges only particular coordination numbers will accommodate the different sized ions contacting each other.

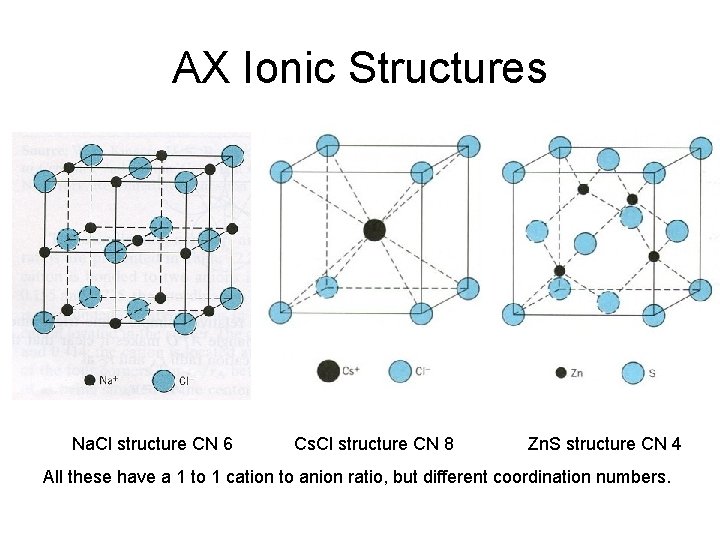
AX Ionic Structures Na. Cl structure CN 6 Cs. Cl structure CN 8 Zn. S structure CN 4 All these have a 1 to 1 cation to anion ratio, but different coordination numbers.

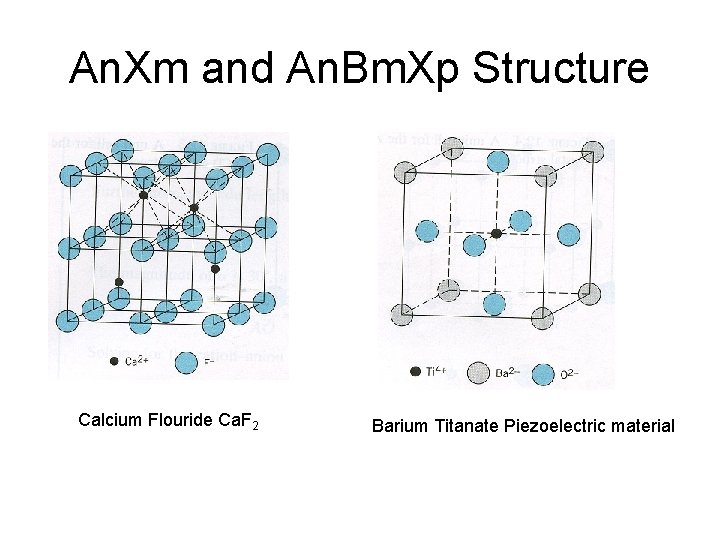
An. Xm and An. Bm. Xp Structure Calcium Flouride Ca. F 2 Barium Titanate Piezoelectric material

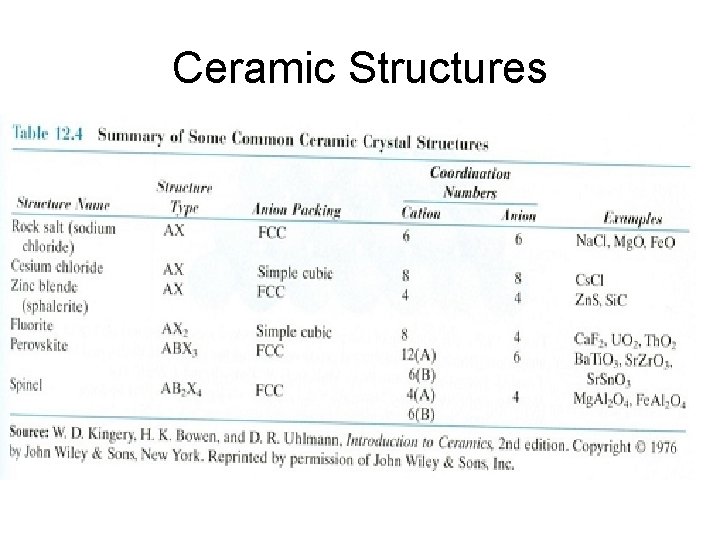
Ceramic Structures

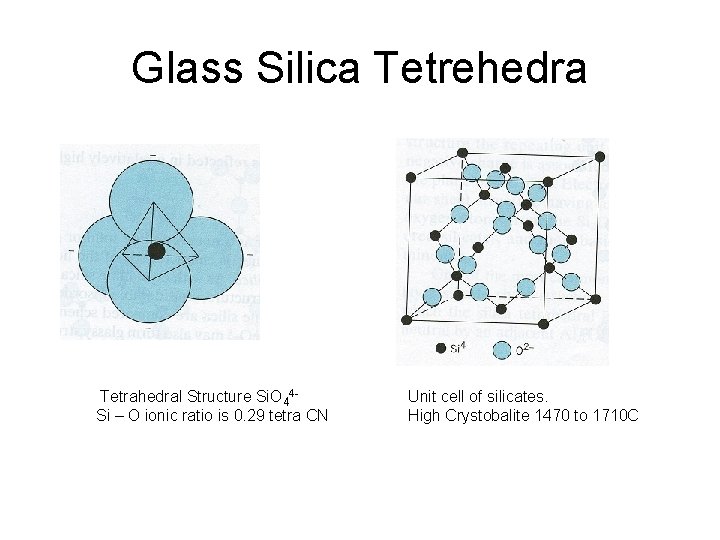
Glass Silica Tetrehedra Tetrahedral Structure Si. O 44 Si – O ionic ratio is 0. 29 tetra CN Unit cell of silicates. High Crystobalite 1470 to 1710 C

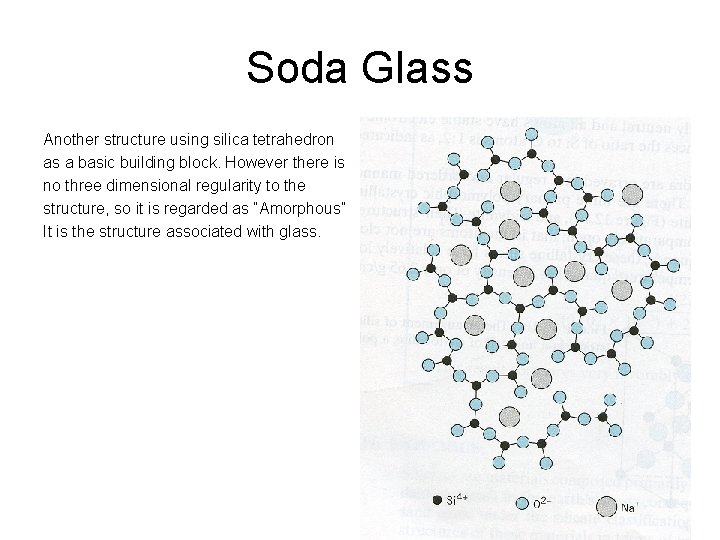
Soda Glass Another structure using silica tetrahedron as a basic building block. However there is no three dimensional regularity to the structure, so it is regarded as “Amorphous” It is the structure associated with glass.

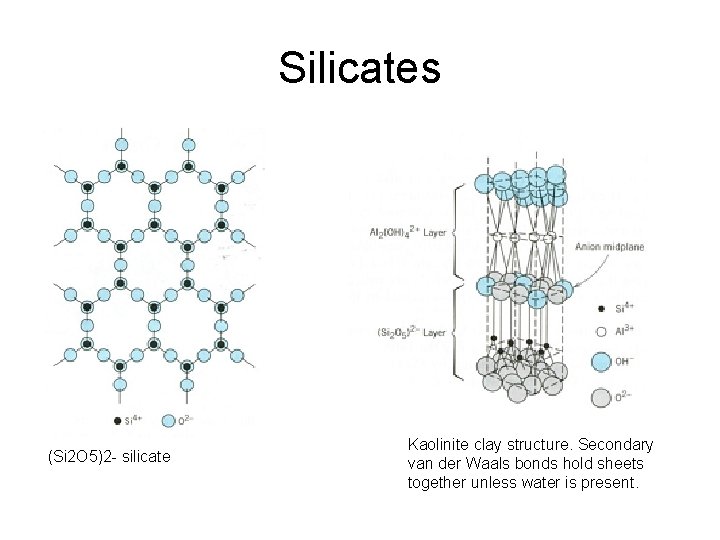
Silicates (Si 2 O 5)2 - silicate Kaolinite clay structure. Secondary van der Waals bonds hold sheets together unless water is present.

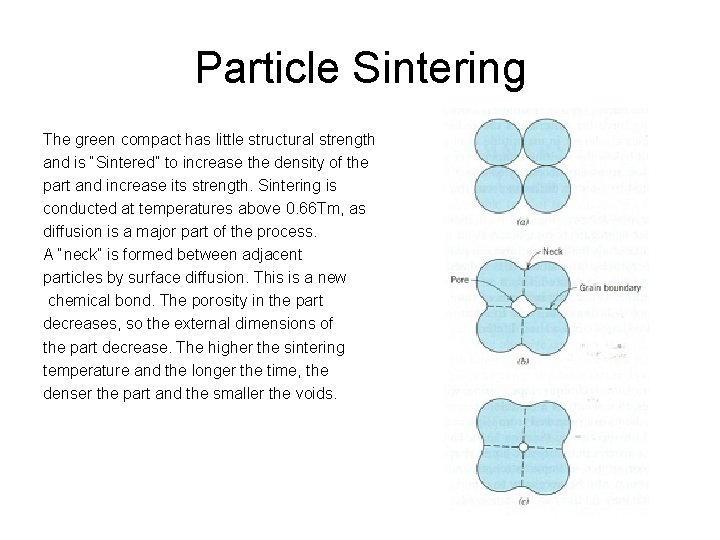
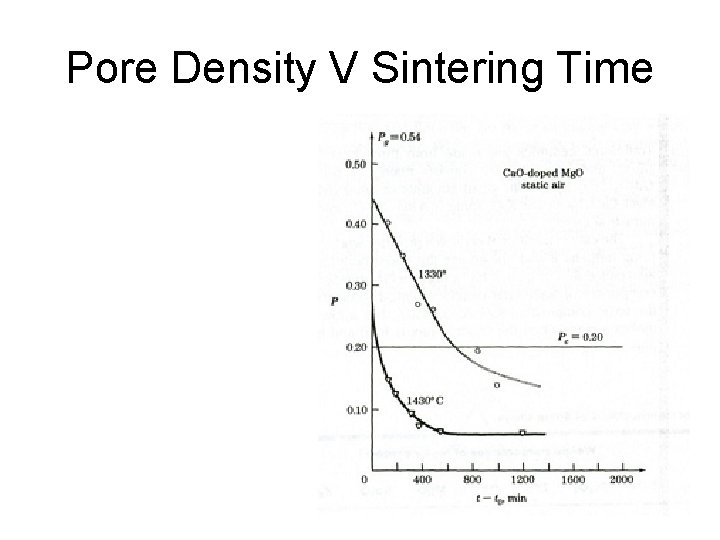
Particle Sintering The green compact has little structural strength and is “Sintered” to increase the density of the part and increase its strength. Sintering is conducted at temperatures above 0. 66 Tm, as diffusion is a major part of the process. A “neck” is formed between adjacent particles by surface diffusion. This is a new chemical bond. The porosity in the part decreases, so the external dimensions of the part decrease. The higher the sintering temperature and the longer the time, the denser the part and the smaller the voids.

Pore Density V Sintering Time

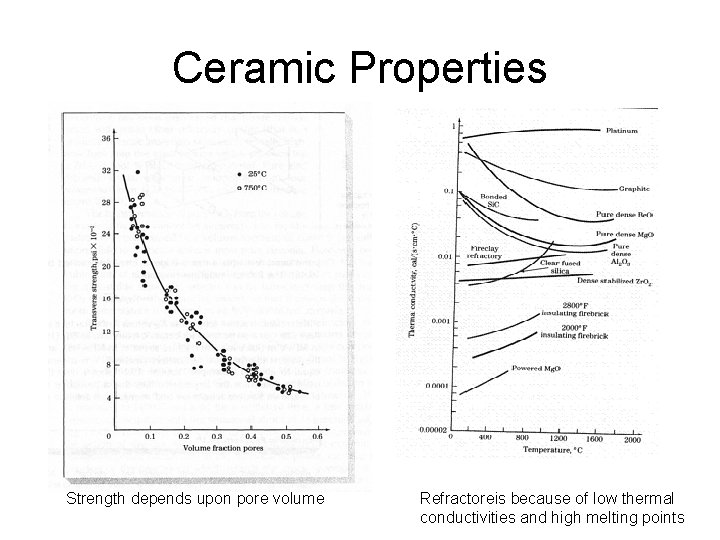
Ceramic Properties Strength depends upon pore volume Refractoreis because of low thermal conductivities and high melting points

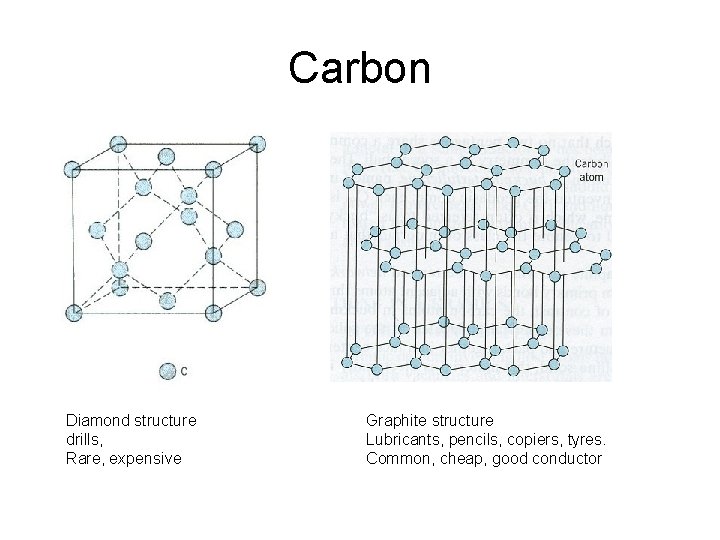
Carbon Diamond structure drills, Rare, expensive Graphite structure Lubricants, pencils, copiers, tyres. Common, cheap, good conductor

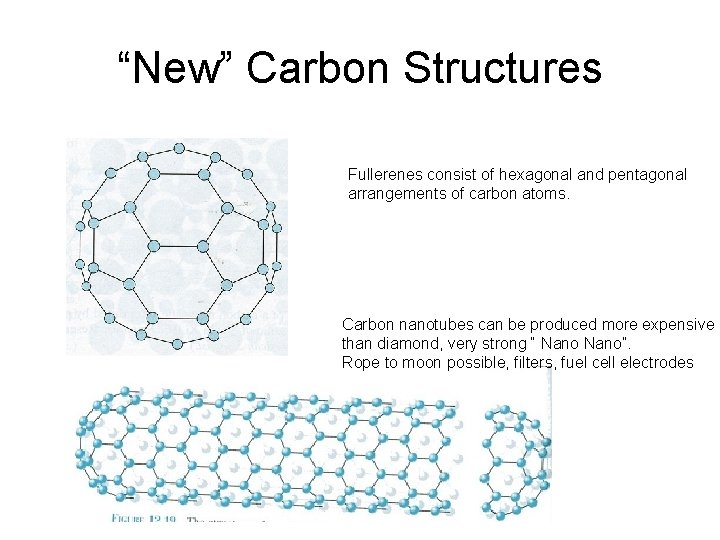
“New” Carbon Structures Fullerenes consist of hexagonal and pentagonal arrangements of carbon atoms. Carbon nanotubes can be produced more expensive than diamond, very strong “ Nano”. Rope to moon possible, filters, fuel cell electrodes

Last Slide Have a good vacation.