CHE 242 Unit VI The Study of Conjugated




![[N]Annulenes • [4]Annulene is antiaromatic (4 N e-’s) • [8]Annulene would be antiaromatic, but [N]Annulenes • [4]Annulene is antiaromatic (4 N e-’s) • [8]Annulene would be antiaromatic, but](https://slidetodoc.com/presentation_image_h2/b25beec27ab99c206c3c82a4d9be6a86/image-10.jpg)



- Slides: 26

CHE 242 Unit VI The Study of Conjugated Systems, Aromaticity, and Reactions of Aromatic Compounds CHAPTER SIXTEEN Terrence P. Sherlock Burlington County College Chapter 16 2004

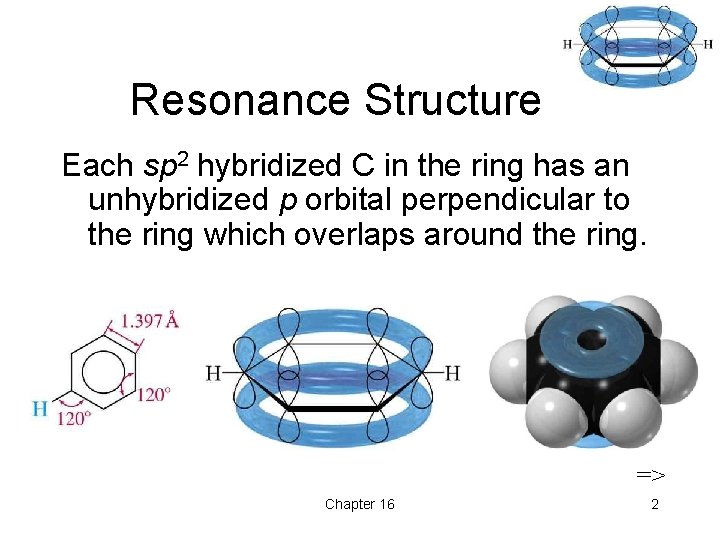
Resonance Structure Each sp 2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. => Chapter 16 2

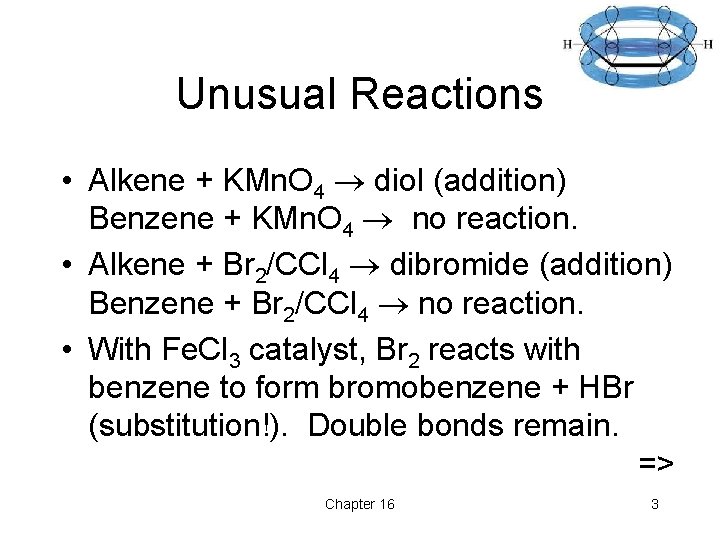
Unusual Reactions • Alkene + KMn. O 4 diol (addition) Benzene + KMn. O 4 no reaction. • Alkene + Br 2/CCl 4 dibromide (addition) Benzene + Br 2/CCl 4 no reaction. • With Fe. Cl 3 catalyst, Br 2 reacts with benzene to form bromobenzene + HBr (substitution!). Double bonds remain. => Chapter 16 3

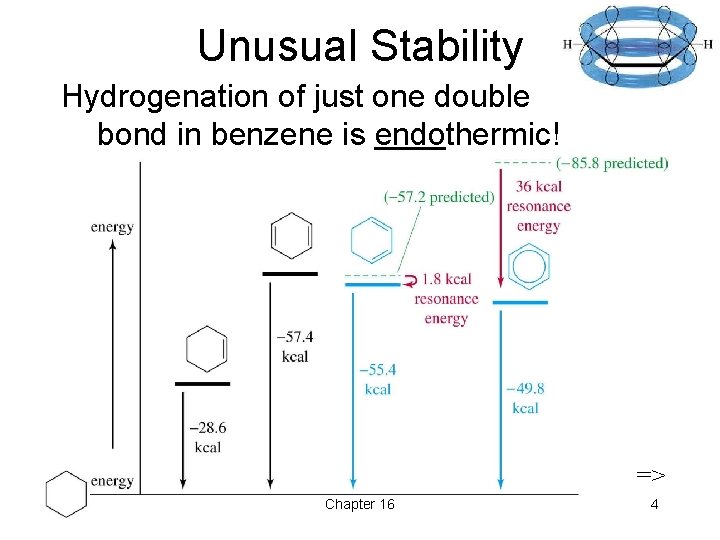
Unusual Stability Hydrogenation of just one double bond in benzene is endothermic! => Chapter 16 4

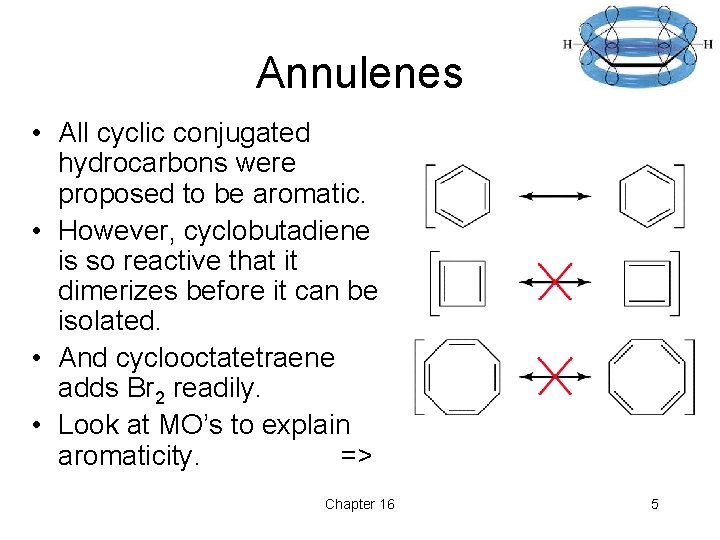
Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • And cyclooctatetraene adds Br 2 readily. • Look at MO’s to explain aromaticity. => Chapter 16 5

MO Rules for Benzene • Six overlapping p orbitals must form six molecular orbitals. • Three will be bonding, three antibonding. • Lowest energy MO will have all bonding interactions, no nodes. • As energy of MO increases, the number of nodes increases. => Chapter 16 6

Aromatic Requirements • Structure must be cyclic with conjugated pi bonds. • Each atom in the ring must have an unhybridized p orbital. • The p orbitals must overlap continuously around the ring. (Usually planar structure) • Compound is more stable than its openchain counterpart. => Chapter 16 7

Anti- and Nonaromatic • Antiaromatic compounds are cyclic, conjugated, with overlapping p orbitals around the ring, but the energy of the compound is greater than its open-chain counterpart. • Nonaromatic compounds do not have a continuous ring of overlapping p orbitals and may be nonplanar. => Chapter 16 8

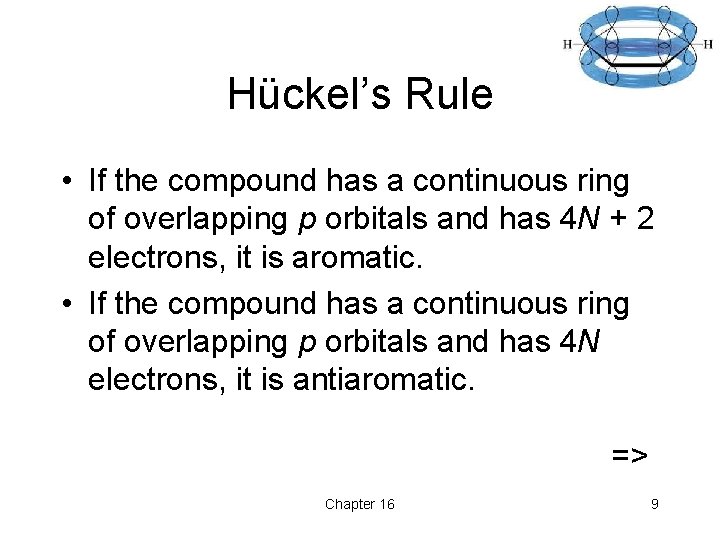
Hückel’s Rule • If the compound has a continuous ring of overlapping p orbitals and has 4 N + 2 electrons, it is aromatic. • If the compound has a continuous ring of overlapping p orbitals and has 4 N electrons, it is antiaromatic. => Chapter 16 9
![NAnnulenes 4Annulene is antiaromatic 4 N es 8Annulene would be antiaromatic but [N]Annulenes • [4]Annulene is antiaromatic (4 N e-’s) • [8]Annulene would be antiaromatic, but](https://slidetodoc.com/presentation_image_h2/b25beec27ab99c206c3c82a4d9be6a86/image-10.jpg)
[N]Annulenes • [4]Annulene is antiaromatic (4 N e-’s) • [8]Annulene would be antiaromatic, but it’s not planar, so it’s nonaromatic. • [10]Annulene is aromatic except for the isomers that are not planar. • Larger 4 N annulenes are not antiaromatic because they are flexible enough to become nonplanar. => Chapter 16 10

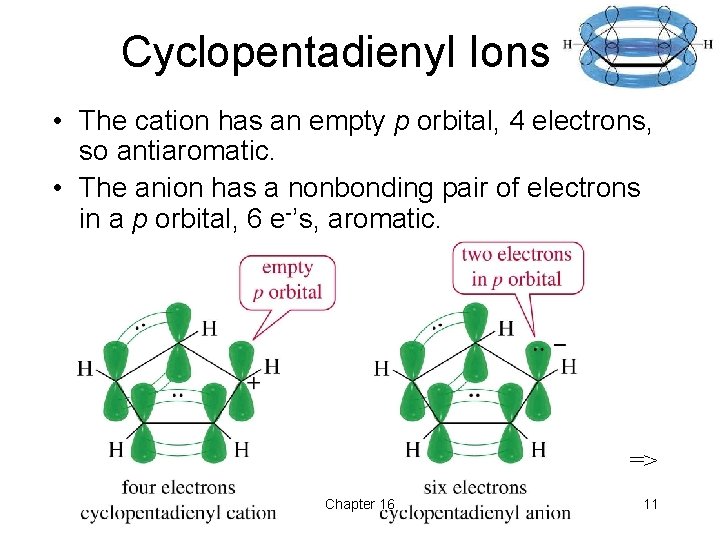
Cyclopentadienyl Ions • The cation has an empty p orbital, 4 electrons, so antiaromatic. • The anion has a nonbonding pair of electrons in a p orbital, 6 e-’s, aromatic. => Chapter 16 11

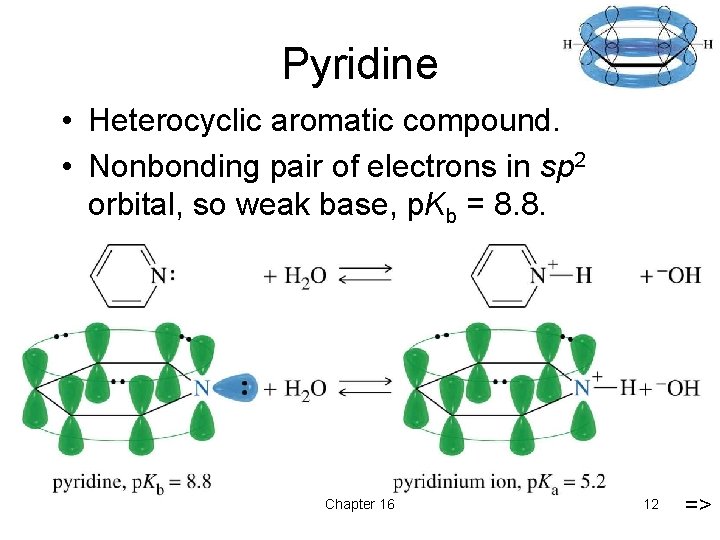
Pyridine • Heterocyclic aromatic compound. • Nonbonding pair of electrons in sp 2 orbital, so weak base, p. Kb = 8. 8. Chapter 16 12 =>

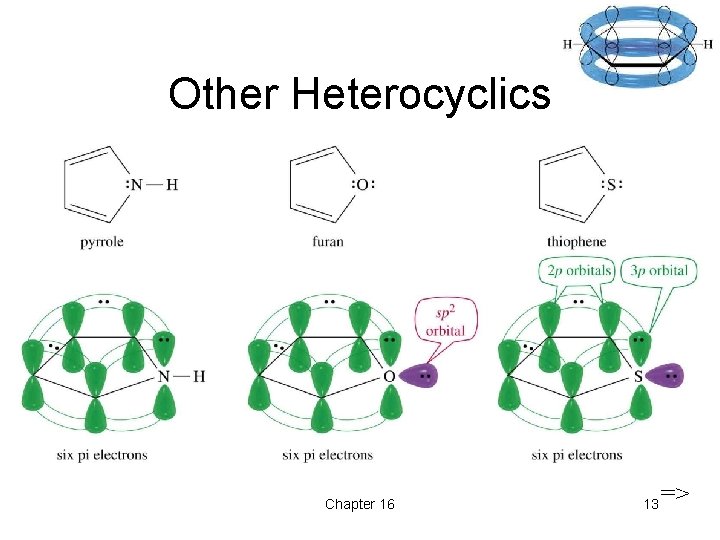
Other Heterocyclics Chapter 16 13 =>

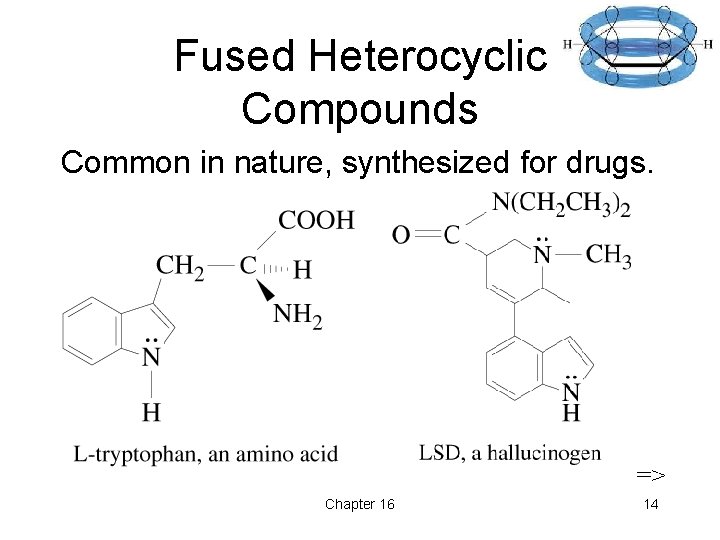
Fused Heterocyclic Compounds Common in nature, synthesized for drugs. => Chapter 16 14

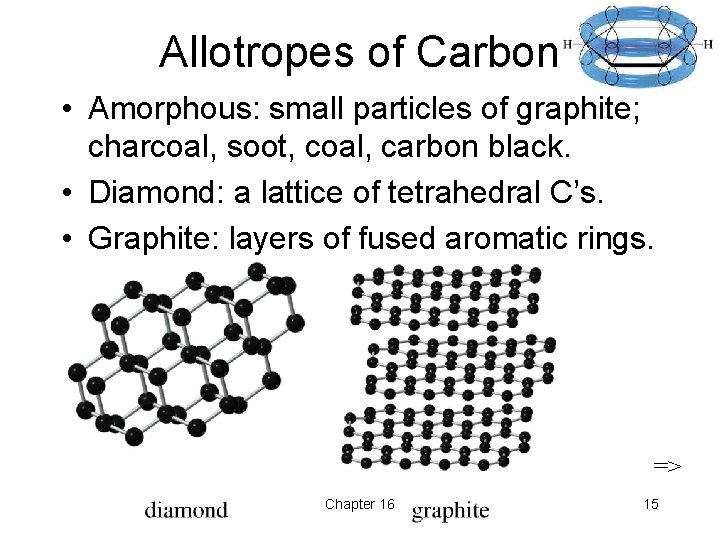
Allotropes of Carbon • Amorphous: small particles of graphite; charcoal, soot, coal, carbon black. • Diamond: a lattice of tetrahedral C’s. • Graphite: layers of fused aromatic rings. => Chapter 16 15

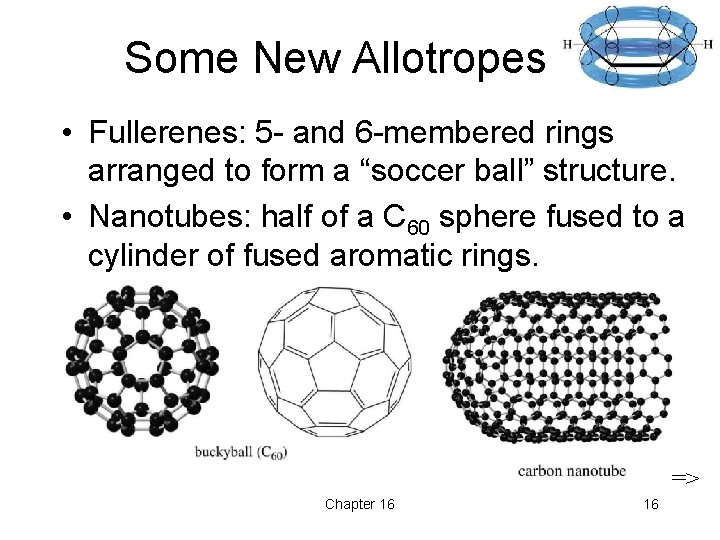
Some New Allotropes • Fullerenes: 5 - and 6 -membered rings arranged to form a “soccer ball” structure. • Nanotubes: half of a C 60 sphere fused to a cylinder of fused aromatic rings. => Chapter 16 16

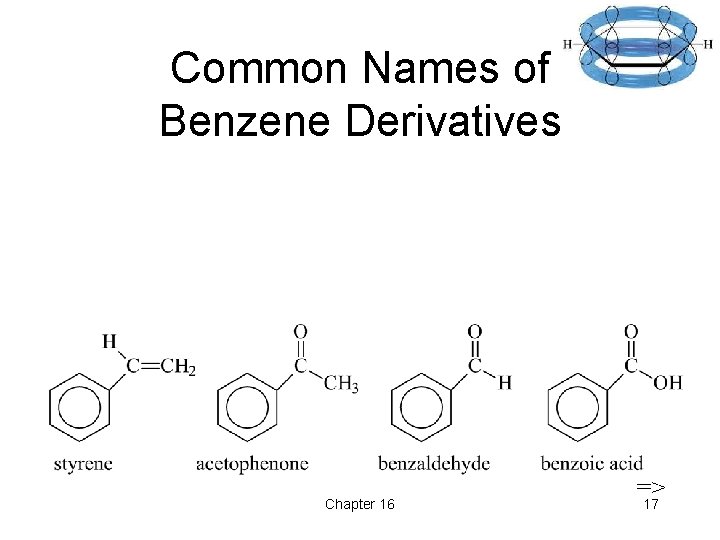
Common Names of Benzene Derivatives Chapter 16 => 17

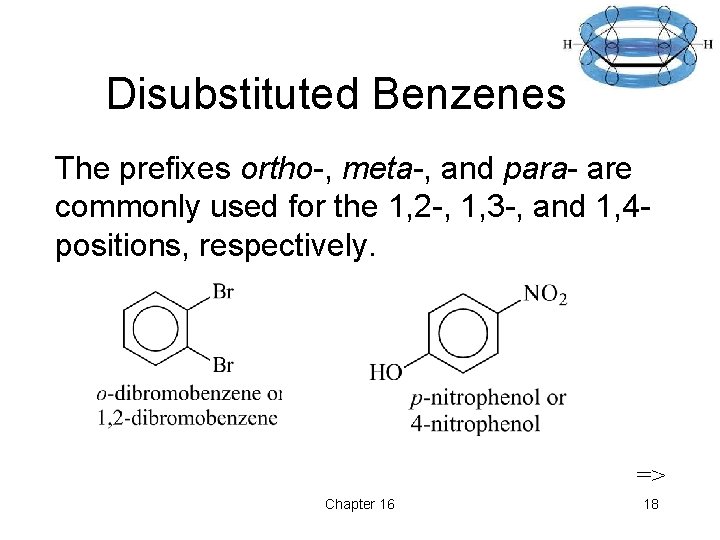
Disubstituted Benzenes The prefixes ortho-, meta-, and para- are commonly used for the 1, 2 -, 1, 3 -, and 1, 4 positions, respectively. => Chapter 16 18

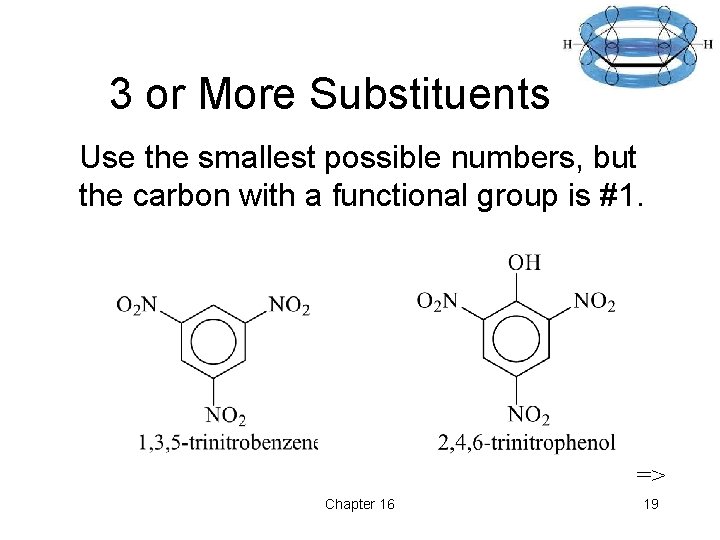
3 or More Substituents Use the smallest possible numbers, but the carbon with a functional group is #1. => Chapter 16 19

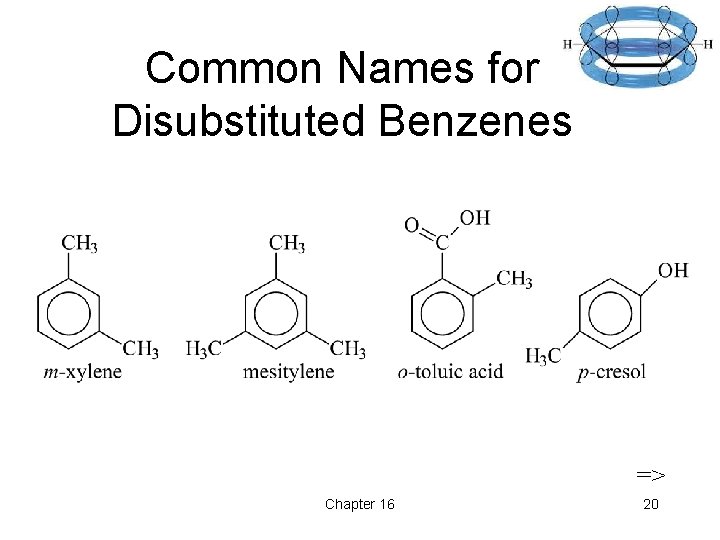
Common Names for Disubstituted Benzenes => Chapter 16 20

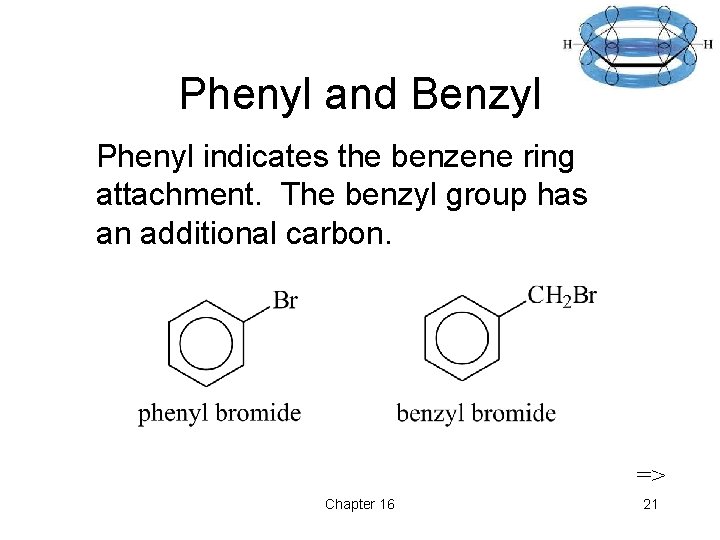
Phenyl and Benzyl Phenyl indicates the benzene ring attachment. The benzyl group has an additional carbon. => Chapter 16 21

Physical Properties • Melting points: More symmetrical than corresponding alkane, pack better into crystals, so higher melting points. • Boiling points: Dependent on dipole moment, so ortho > meta > para, for disubstituted benzenes. • Density: More dense than nonaromatics, less dense than water. • Solubility: Generally insoluble in water. => Chapter 16 22

IR and NMR Spectroscopy • C=C stretch absorption at 1600 cm-1. • sp 2 C-H stretch just above 3000 cm-1. • 1 H NMR at 7 - 8 for H’s on aromatic ring. • 13 C NMR at 120 - 150, similar to alkene carbons. => Chapter 16 23

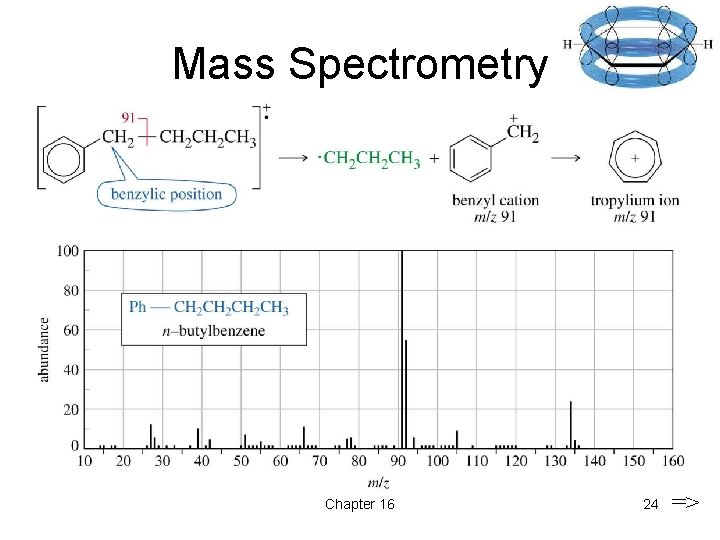
Mass Spectrometry => Chapter 16 24 =>

Chapter 16 25

POWER POINT IMAGES FROM “ORGANIC CHEMISTRY, 5 TH EDITION” L. G. WADE ALL MATERIALS USED WITH PERMISSION OF AUTHOR PRESENTATION ADAPTED FOR BURLINGTON COUNTY COLLEGE ORGANIC CHEMISTRY COURSE BY: ANNALICIA POEHLER STEFANIE LAYMAN CALY MARTIN Chapter 16 26