CHE 205 Stoichiometry Reactive Systems Flowcharts and DOF


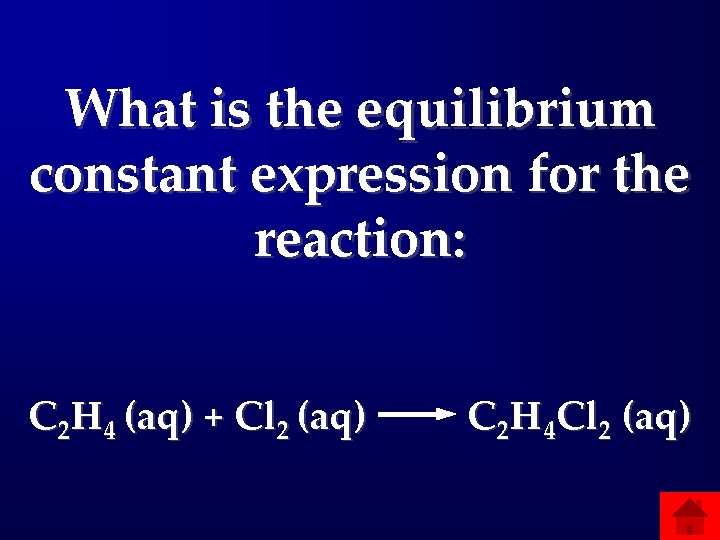










- Slides: 28

CHE 205

Stoichiometry Reactive Systems Flowcharts and DOF Balances Empty $200 $200 $400 $400 $600 $600 $800 $800 $1000 $1000

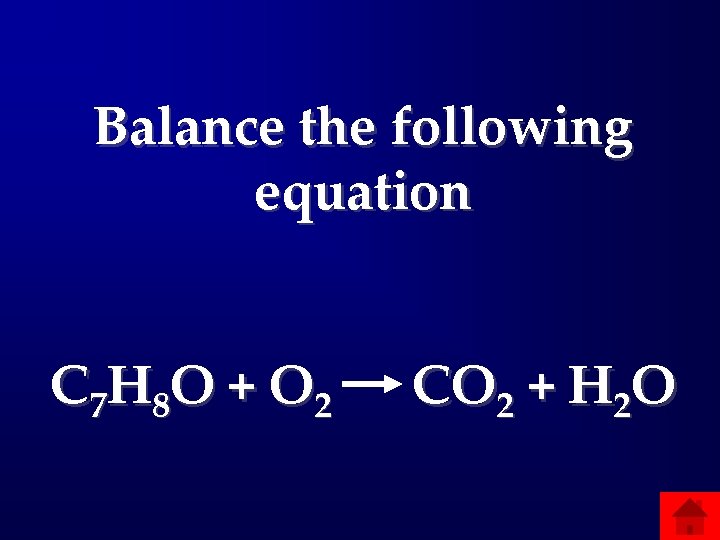
Balance the following equation C 7 H 8 O + O 2 CO 2 + H 2 O

What is the fractional conversion if 50 lbm of propane are fed to a reactor and 15 lbm are in the product
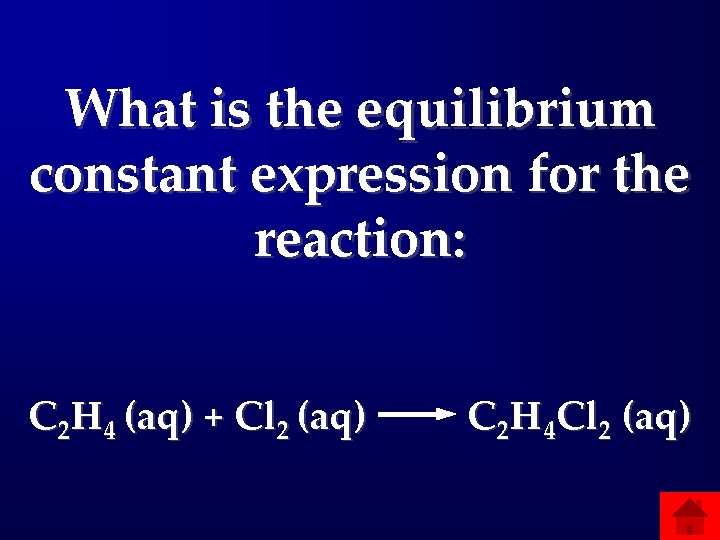
What is the equilibrium constant expression for the reaction: C 2 H 4 (aq) + Cl 2 (aq) C 2 H 4 Cl 2 (aq)

How much air is needed per mole of propane is completely combusted in the presence of 60% excess air

Write all of the atomic balances for the following flow sheet 100 moles 0. 25 mol C 3 H 6/mol 0. 10 mol NH 3/mol 0. 65 mol air/mol n 1 mol C 3 H 6 n 2 mol NH 3 n 3 mol O 2 n 4 mol N 2 n 5 mol C 3 H 3 N n 6 mol H 2 O

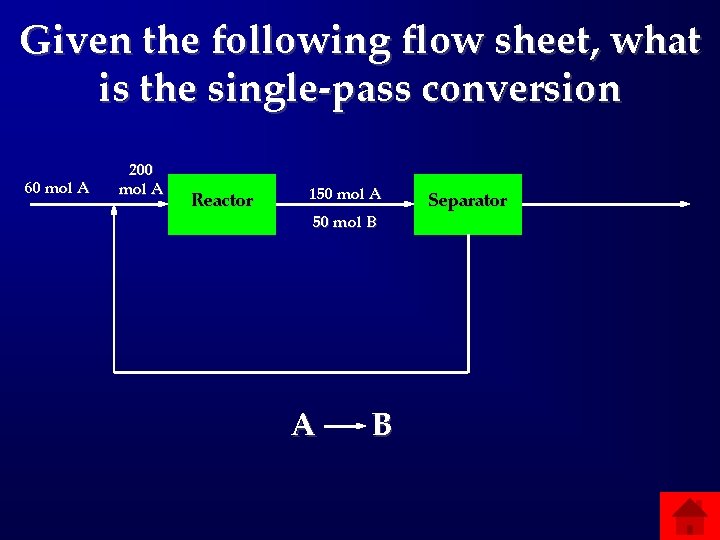
Given the following flow sheet, what is the single-pass conversion 60 mol A 200 mol A Reactor 150 mol A 50 mol B A B Separator

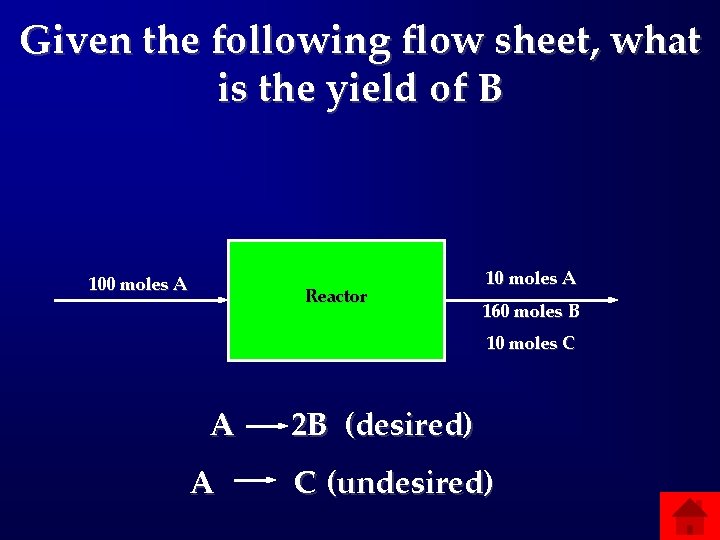
Given the following flow sheet, what is the yield of B 100 moles A Reactor 10 moles A 160 moles B 10 moles C A A 2 B (desired) C (undesired)

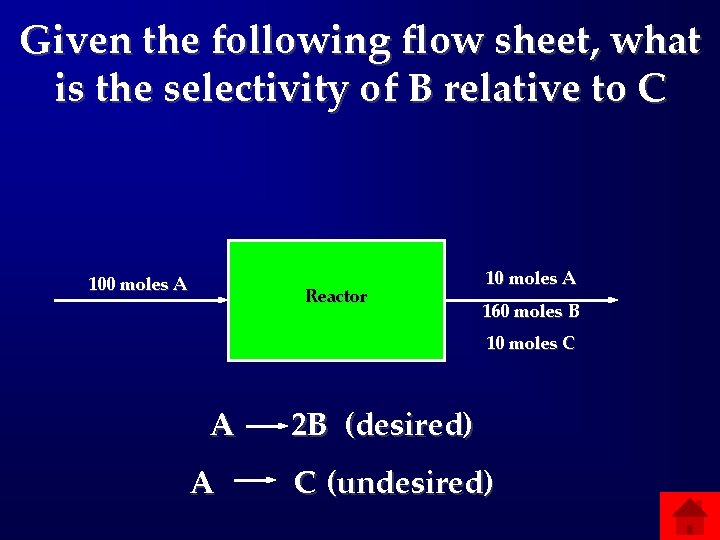
Given the following flow sheet, what is the selectivity of B relative to C 100 moles A Reactor 10 moles A 160 moles B 10 moles C A A 2 B (desired) C (undesired)

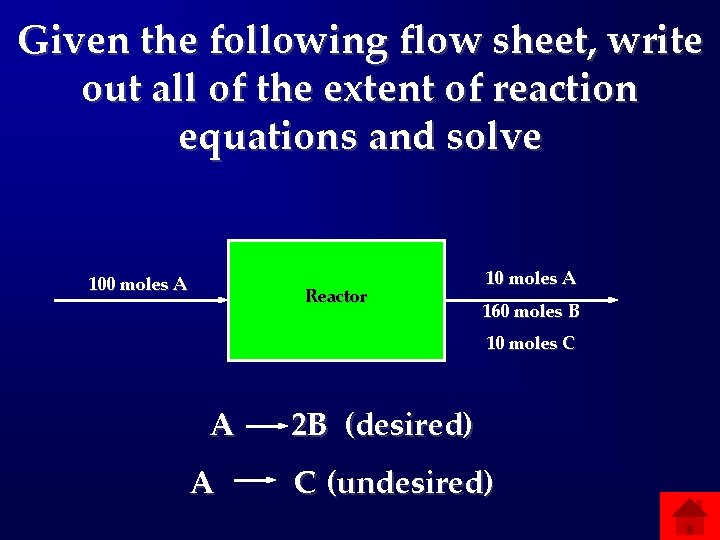
Given the following flow sheet, write out all of the extent of reaction equations and solve 100 moles A Reactor 10 moles A 160 moles B 10 moles C A A 2 B (desired) C (undesired)

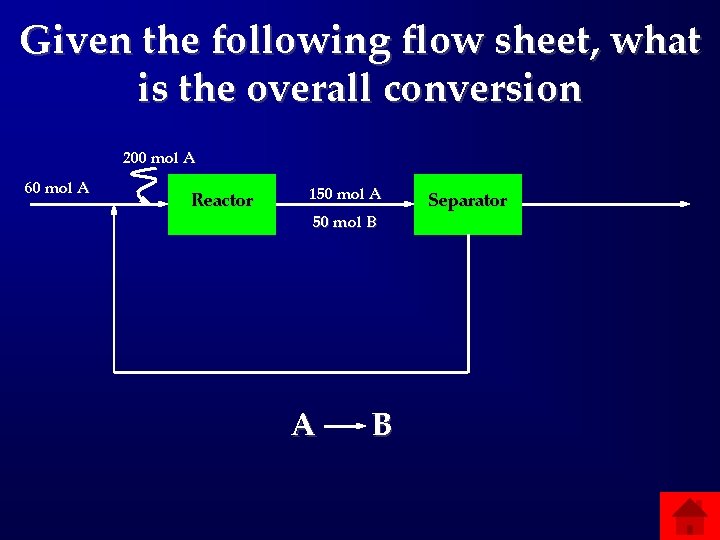
Given the following flow sheet, what is the overall conversion 200 mol A 60 mol A Reactor 150 mol A 50 mol B A B Separator

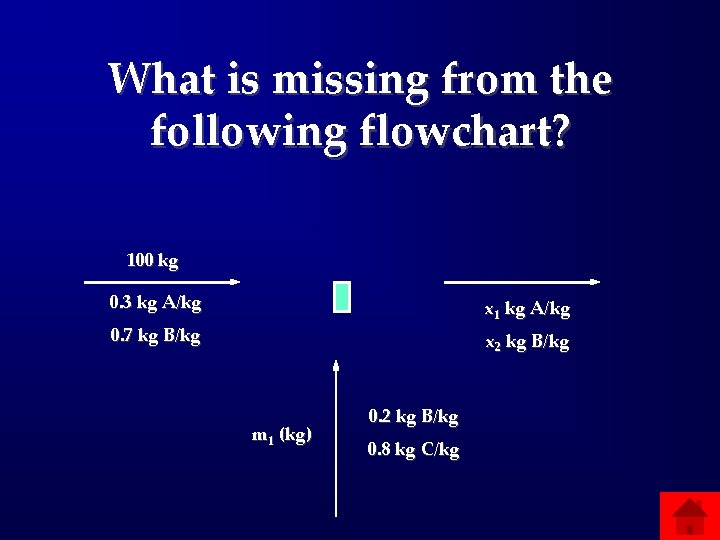
What is missing from the following flowchart? 100 kg 0. 3 kg A/kg x 1 kg A/kg 0. 7 kg B/kg x 2 kg B/kg m 1 (kg) 0. 2 kg B/kg 0. 8 kg C/kg

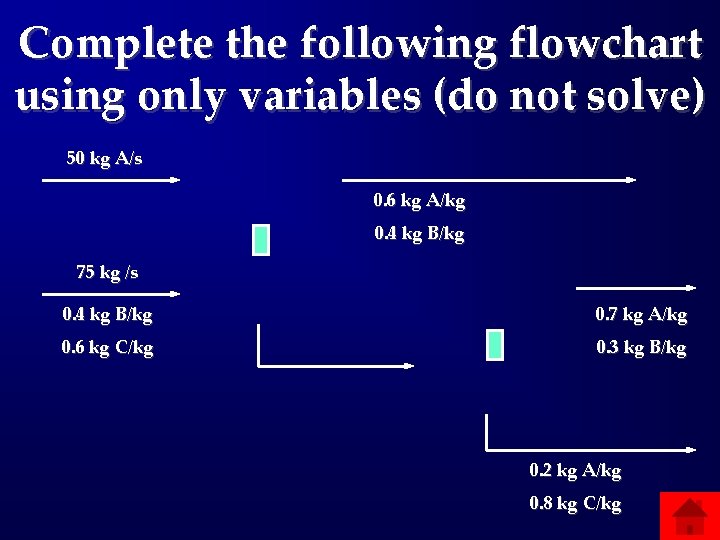
Complete the following flowchart using only variables (do not solve) 50 kg A/s 0. 6 kg A/kg 0. 4 kg B/kg 75 kg /s 0. 4 kg B/kg 0. 7 kg A/kg 0. 6 kg C/kg 0. 3 kg B/kg 0. 2 kg A/kg 0. 8 kg C/kg

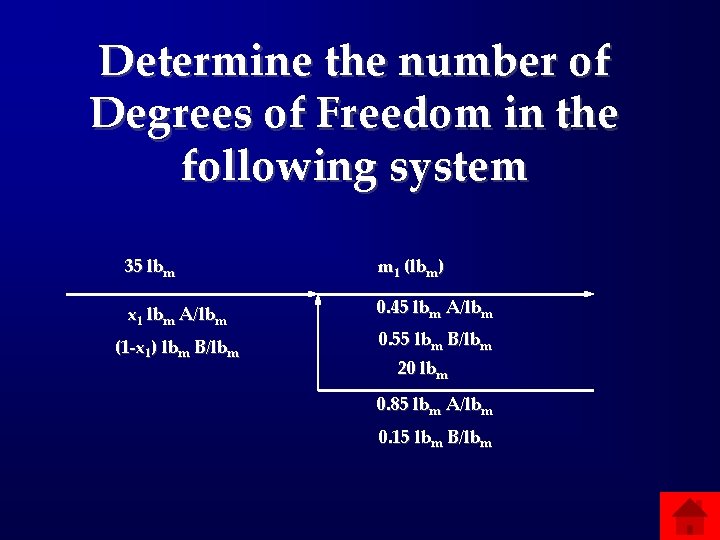
Determine the number of Degrees of Freedom in the following system 35 lbm m 1 (lbm) x 1 lbm A/lbm 0. 45 lbm A/lbm (1 -x 1) lbm B/lbm 0. 55 lbm B/lbm 20 lbm 0. 85 lbm A/lbm 0. 15 lbm B/lbm

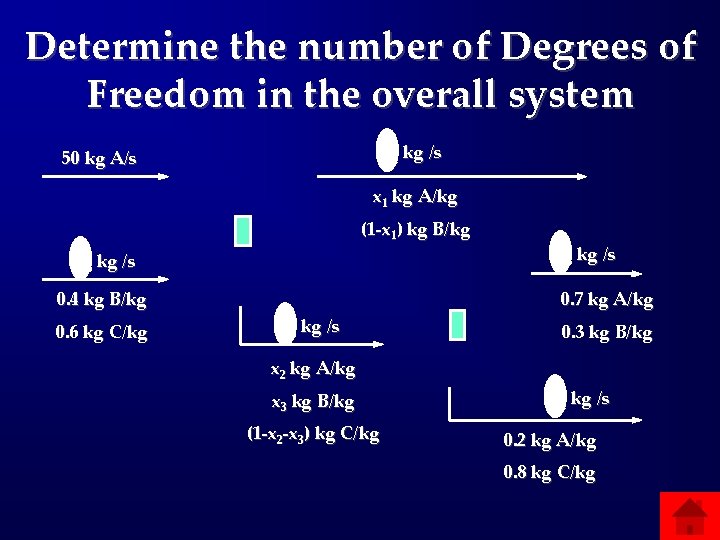
Determine the number of Degrees of Freedom in the overall system m 1 kg /s 50 kg A/s x 1 kg A/kg (1 -x 1) kg B/kg mo kg /s 0. 4 kg B/kg 0. 6 kg C/kg m 3 kg /s 0. 7 kg A/kg m 2 kg /s 0. 3 kg B/kg x 2 kg A/kg x 3 kg B/kg (1 -x 2 -x 3) kg C/kg m 4 kg /s 0. 2 kg A/kg 0. 8 kg C/kg


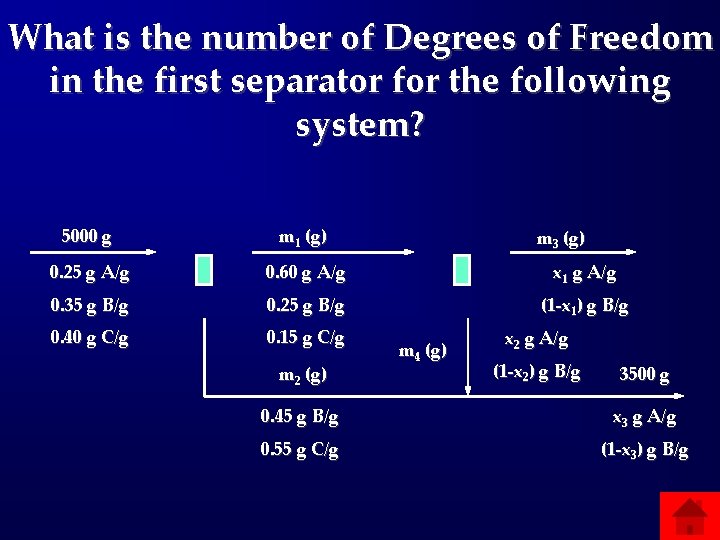
What is the number of Degrees of Freedom in the first separator for the following system? 5000 g m 1 (g) 0. 25 g A/g 0. 60 g A/g x 1 g A/g 0. 35 g B/g 0. 25 g B/g (1 -x 1) g B/g 0. 40 g C/g 0. 15 g C/g m 2 (g) m 3 (g) m 4 (g) x 2 g A/g (1 -x 2) g B/g 3500 g 0. 45 g B/g x 3 g A/g 0. 55 g C/g (1 -x 3) g B/g

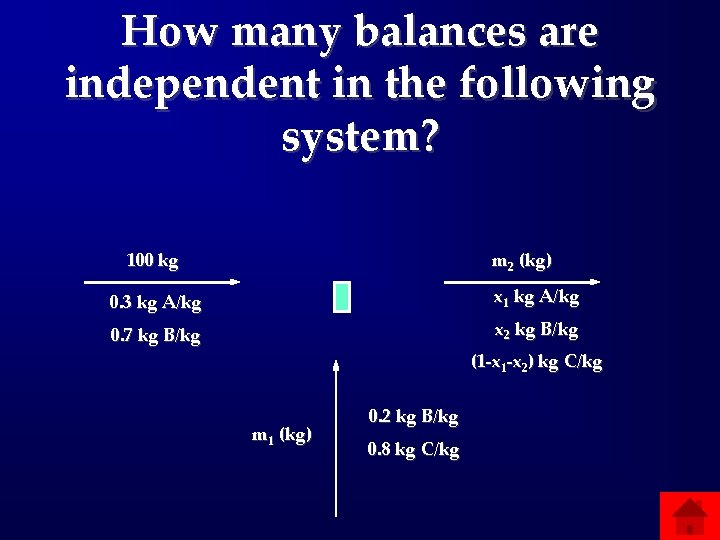
How many balances are independent in the following system? 100 kg m 2 (kg) 0. 3 kg A/kg x 1 kg A/kg 0. 7 kg B/kg x 2 kg B/kg (1 -x 2) kg C/kg m 1 (kg) 0. 2 kg B/kg 0. 8 kg C/kg

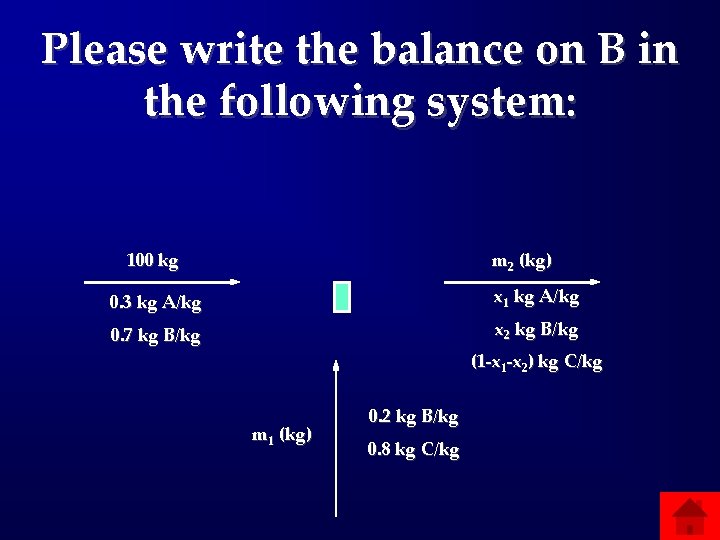
Please write the balance on B in the following system: 100 kg m 2 (kg) 0. 3 kg A/kg x 1 kg A/kg 0. 7 kg B/kg x 2 kg B/kg (1 -x 2) kg C/kg m 1 (kg) 0. 2 kg B/kg 0. 8 kg C/kg

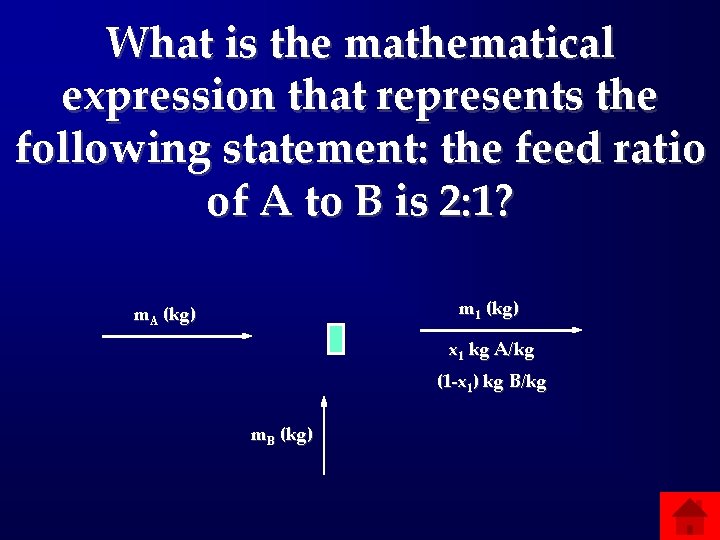
What is the mathematical expression that represents the following statement: the feed ratio of A to B is 2: 1? m 1 (kg) m. A (kg) x 1 kg A/kg (1 -x 1) kg B/kg m. B (kg)


Write the mathematical expression that represents the statement – 70% of the water is stripped from the feed solution and sent to a processing plant m 2 (kg) mo (kg) x 1 kg A/kg x 2 kg A/kg (1 -x 1) kg H 2 O/kg (1 -x 2) kg H 2 O/kg m 1 (kg H 2 O) To processing plant

What is the total molar amount of the overhead product (m. O) if 93% of A is recovered in this stream? 200 mol m. O (mol) 0. 5 mol A/mol 0. 95 mol A/mol 0. 5 mol B/mol 0. 05 mol B/mol m. B (mol) x 1 mol A/mol (1 -x 1) mol B/mol

Answers: Stoichiometry • 200: C 7 H 8 O+(17/2)O 2 7 CO 2+4 H 2 O • 400: 0. 7 mol reacted/mol fed • 600: K=y. C 2 H 4 Cl 2/(y. C 2 H 4 y. Cl 2) • 800: 38. 01 mol Air/mol C 3 H 8 • 1000: C: 25=n 1+n 5 H: 180=6 n 1+3 n 2+3 n 5+2 n 6 N: 112. 7=6 n 1+3 n 2+3 n 5+2 n 6 O: 27. 3=2 n 3+n 6

Answers: Reactive Systems • • • 200: 0. 25 400: Xi 1=80 mols, Xi 2=10 mols 600: 0. 8 800: 16 mol B formed/mol C formed 1000: 0. 833 mol reacted/ mol fed

Answers: Flowcharts and DOF • 200: Total mass of the stream leaving • 400: Add total mass to all exit steams and label middle stream • 600: 0 DOF • 800: Around whole system 1 DOF; Whole system 2 DOF • 1000: -1 DOF

Answers: Balances • • • 200: 3 400: 70+0. 2 m 1=m 2 x 2 600: m. A=2 m. B 800: 0. 7 m 0(1 -x 1)=m 1 1000: 97. 90 mol