CHE 111 Fall 2020 Lecture 5 a Valence





- Slides: 26

CHE 111 Fall 2020 Lecture 5 a Valence Bond Theory (VBT) 1. 2. 3. 4. 5. 6. Overview/Topics Flaw in Lewis Theory Advantages of VBT Formation of sp 3, sp 2, sp orbitals a. Energy Level Diagrams b. Orbital Sketch Formation of Double and Triple Bonds Difference between σ and π bonds Flaws in VBT Theory Read OER – Chapter 5. 1 -5. 3 Skills to Master 1. Homework 5 a Additional Useful Links q www. chemhaven. org/che 111 q Khan Academy q Google/You Tube

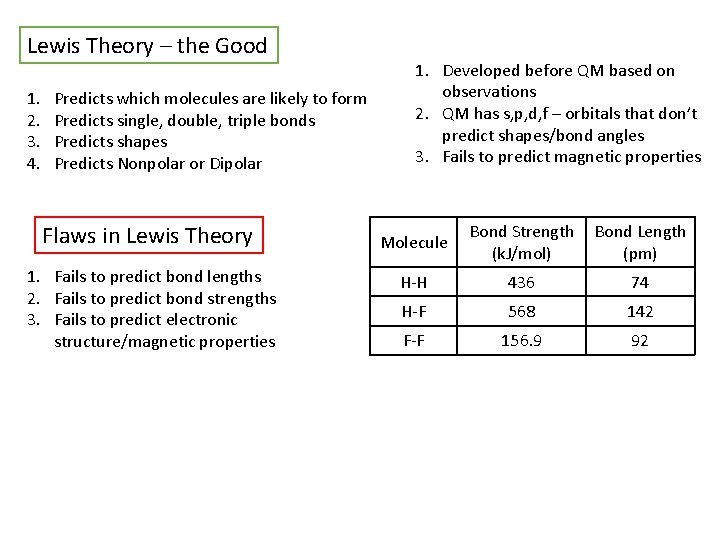
Lewis Theory – the Good 1. 2. 3. 4. Predicts which molecules are likely to form Predicts single, double, triple bonds Predicts shapes Predicts Nonpolar or Dipolar Flaws in Lewis Theory 1. Fails to predict bond lengths 2. Fails to predict bond strengths 3. Fails to predict electronic structure/magnetic properties 1. Developed before QM based on observations 2. QM has s, p, d, f – orbitals that don’t predict shapes/bond angles 3. Fails to predict magnetic properties Molecule Bond Strength (k. J/mol) Bond Length (pm) H-H 436 74 H-F 568 142 F-F 156. 9 92

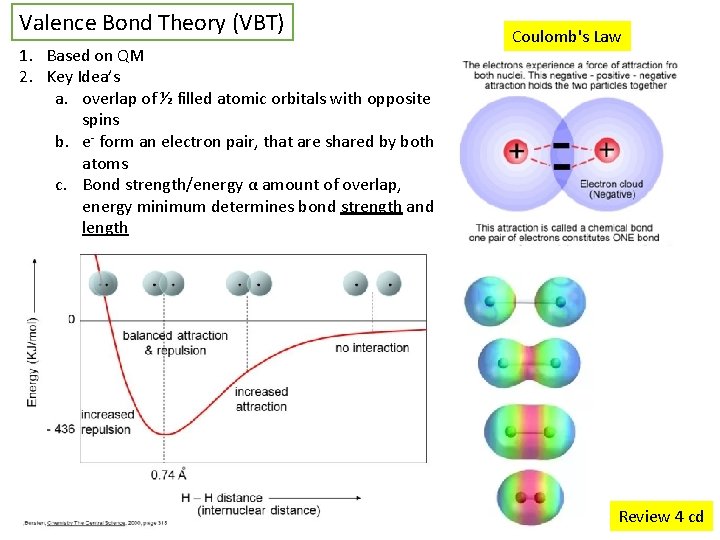
Valence Bond Theory (VBT) 1. Based on QM 2. Key Idea’s a. overlap of ½ filled atomic orbitals with opposite spins b. e- form an electron pair, that are shared by both atoms c. Bond strength/energy α amount of overlap, energy minimum determines bond strength and length Coulomb's Law Review 4 cd

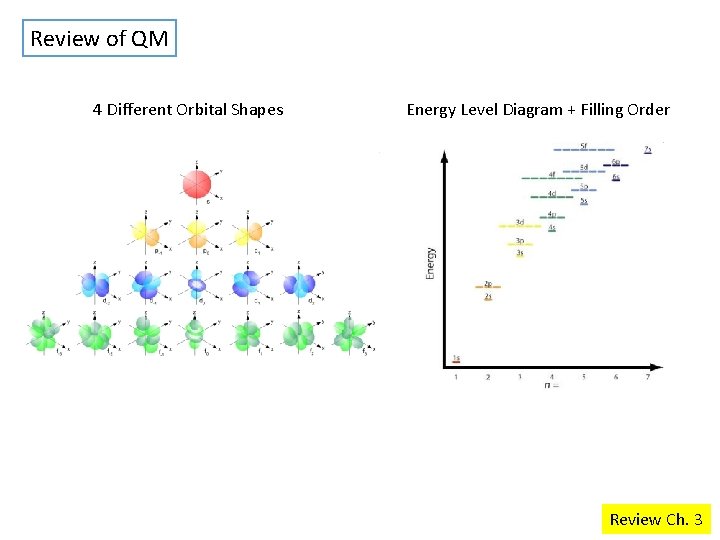
Review of QM 4 Different Orbital Shapes Energy Level Diagram + Filling Order Review Ch. 3

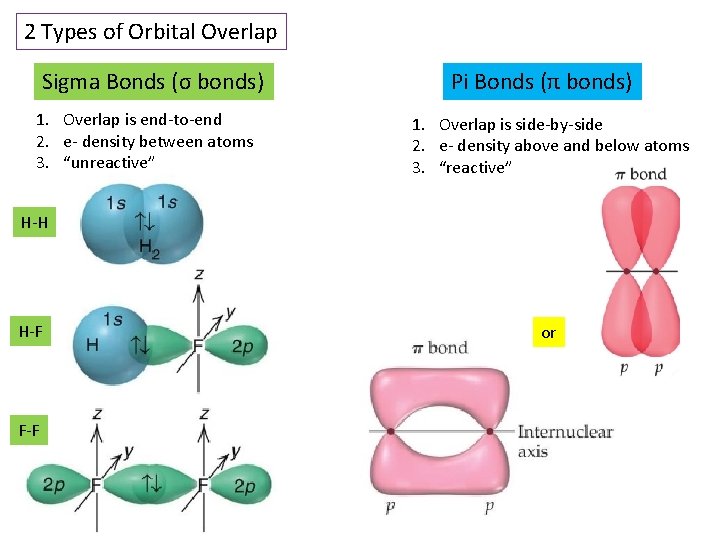
2 Types of Orbital Overlap Sigma Bonds (σ bonds) 1. Overlap is end-to-end 2. e- density between atoms 3. “unreactive” Pi Bonds (π bonds) 1. Overlap is side-by-side 2. e- density above and below atoms 3. “reactive” H-H H-F F-F or

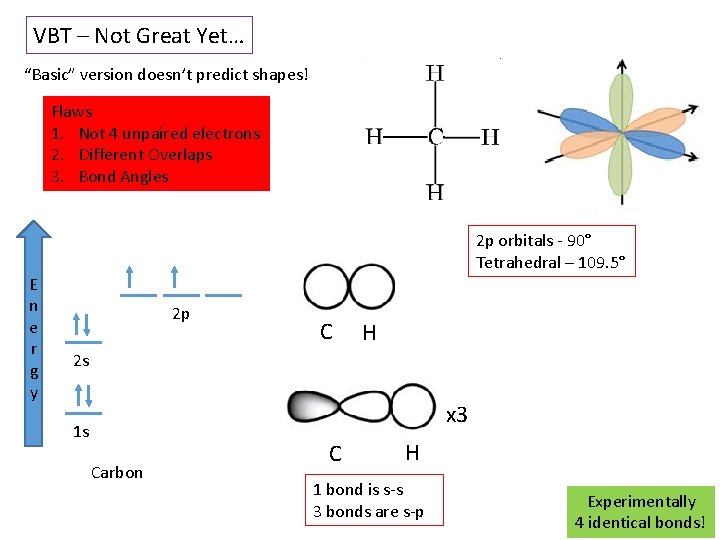
VBT – Not Great Yet… “Basic” version doesn’t predict shapes! Flaws 1. Not 4 unpaired electrons 2. Different Overlaps 3. Bond Angles E n e r g y 2 p orbitals - 90° Tetrahedral – 109. 5° 2 p C H 2 s x 3 1 s Carbon C H 1 bond is s-s 3 bonds are s-p Experimentally 4 identical bonds!

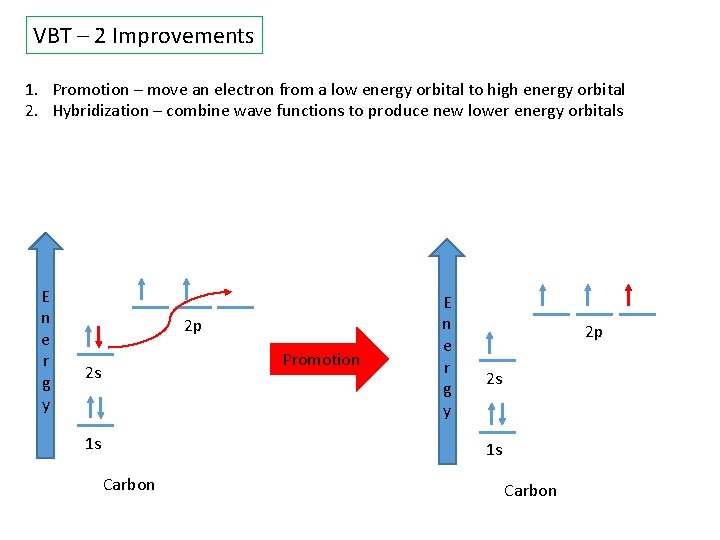
VBT – 2 Improvements 1. Promotion – move an electron from a low energy orbital to high energy orbital 2. Hybridization – combine wave functions to produce new lower energy orbitals E n e r g y 2 p Promotion 2 s 1 s E n e r g y 2 p 2 s 1 s Carbon

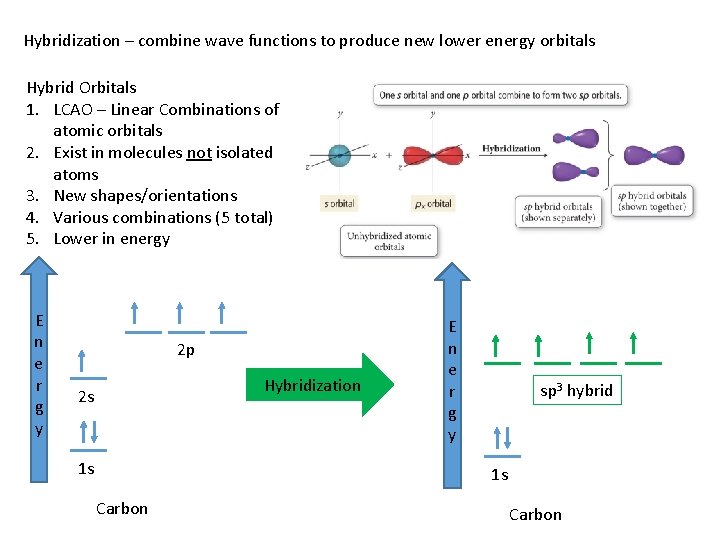
Hybridization – combine wave functions to produce new lower energy orbitals Hybrid Orbitals 1. LCAO – Linear Combinations of atomic orbitals 2. Exist in molecules not isolated atoms 3. New shapes/orientations 4. Various combinations (5 total) 5. Lower in energy E n e r g y 2 p Hybridization 2 s 1 s E n e r g y sp 3 hybrid 1 s Carbon

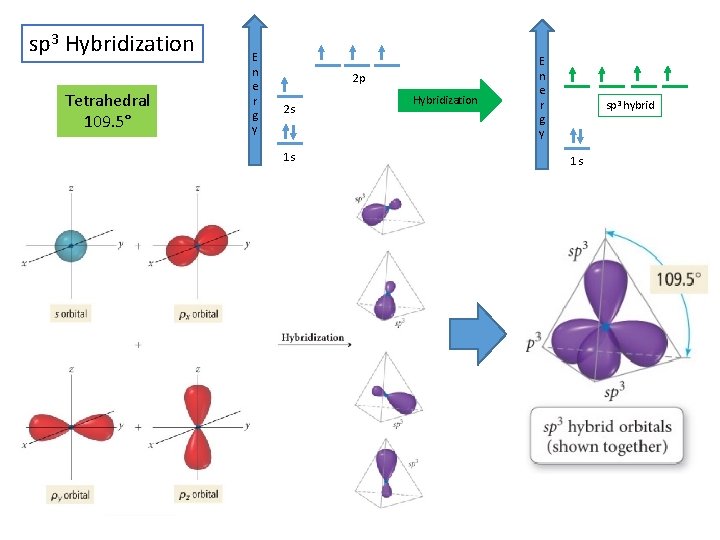
sp 3 Hybridization Tetrahedral 109. 5° E n e r g y 2 p 2 s 1 s Hybridization E n e r g y sp 3 hybrid 1 s

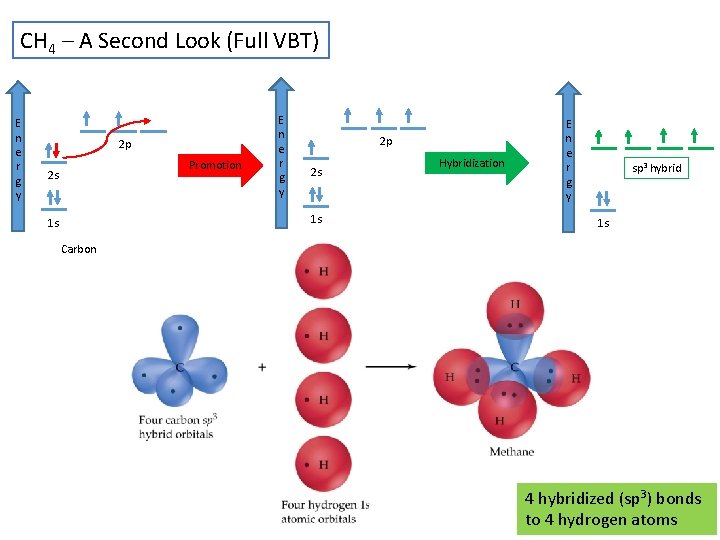
CH 4 – A Second Look (Full VBT) E n e r g y 2 p Promotion 2 s E n e r g y 2 p 2 s 1 s 1 s Hybridization E n e r g y sp 3 hybrid 1 s Carbon 4 hybridized (sp 3) bonds to 4 hydrogen atoms

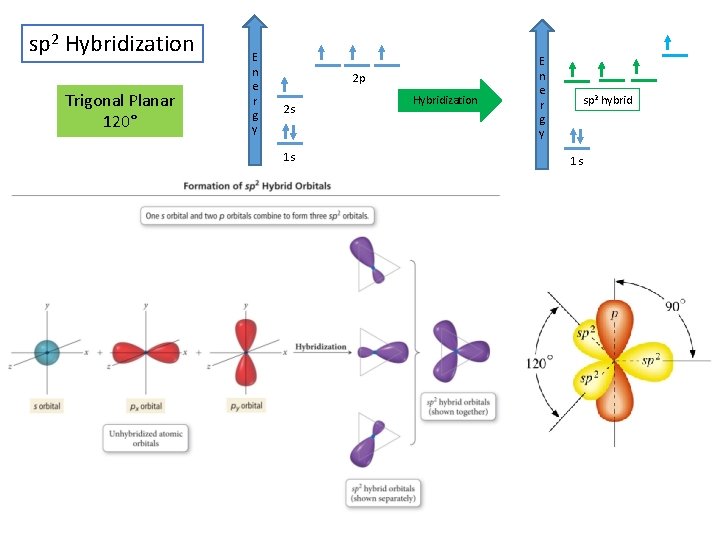
sp 2 Hybridization Trigonal Planar 120° E n e r g y 2 p 2 s 1 s Hybridization E n e r g y sp 2 hybrid 1 s

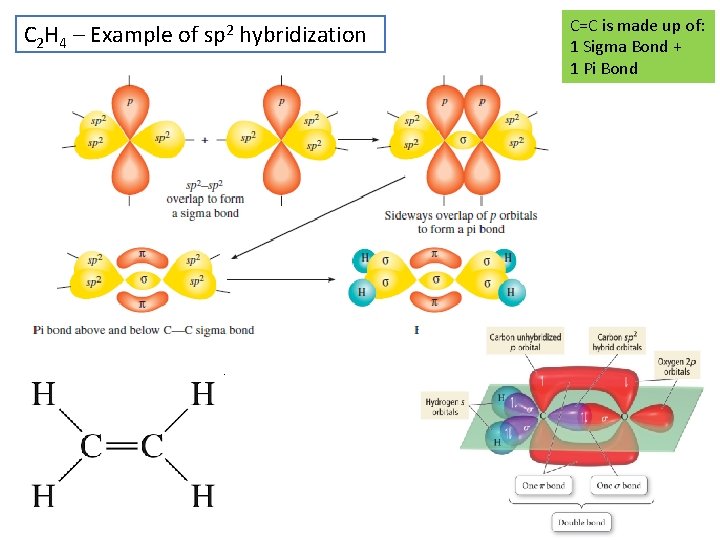
C 2 H 4 – Example of sp 2 hybridization C=C is made up of: 1 Sigma Bond + 1 Pi Bond


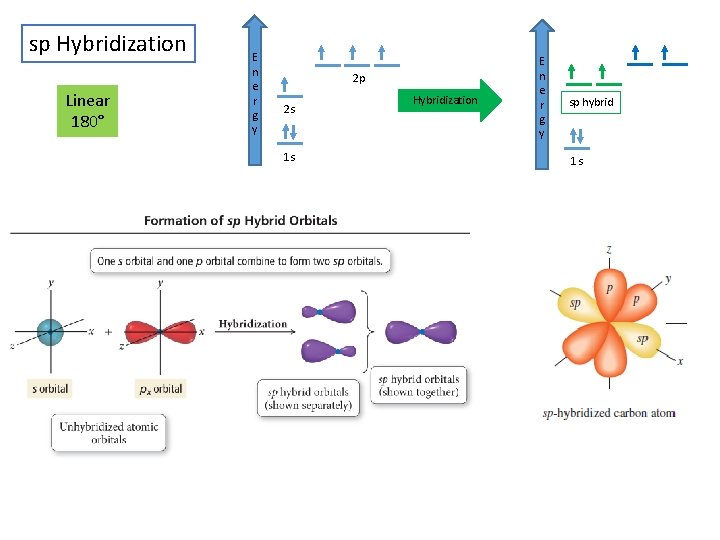
sp Hybridization Linear 180° E n e r g y 2 p 2 s 1 s Hybridization E n e r g y sp hybrid 1 s

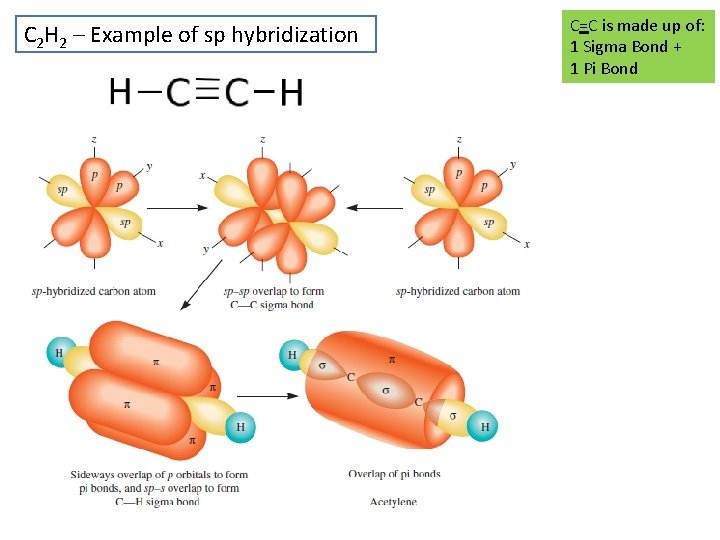
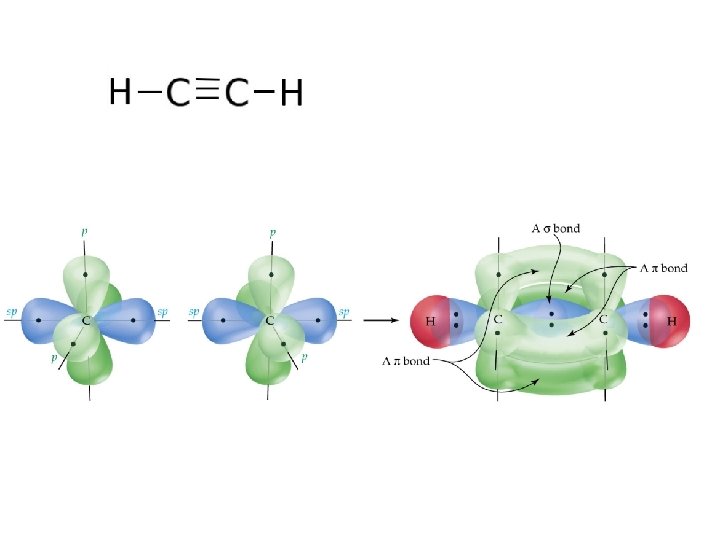
C 2 H 2 – Example of sp hybridization C=C is made up of: 1 Sigma Bond + 1 Pi Bond


Single vs Double vs Triple Bonds sp 3 – hybridization sp 2 – hybridization sp – hybridization

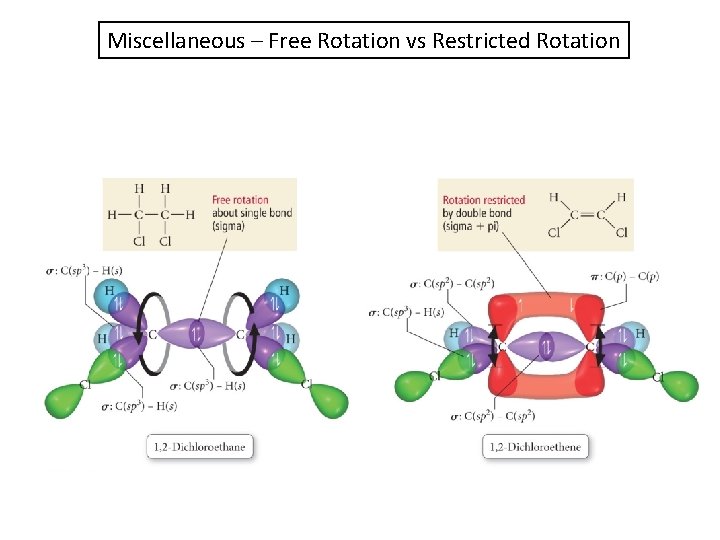
Miscellaneous – Free Rotation vs Restricted Rotation

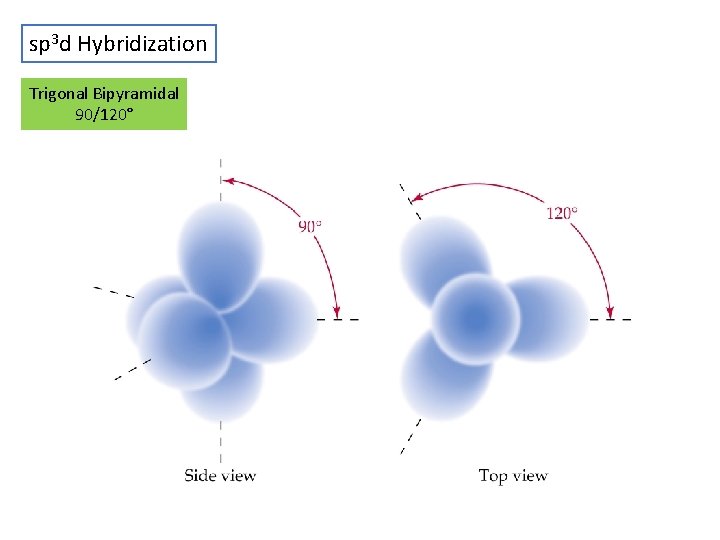
sp 3 d Hybridization Trigonal Bipyramidal 90/120°

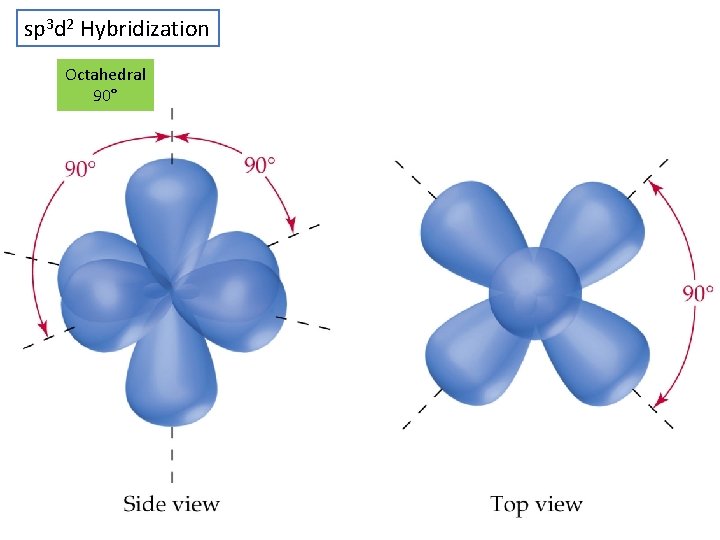
sp 3 d 2 Hybridization Octahedral 90° CHE 111

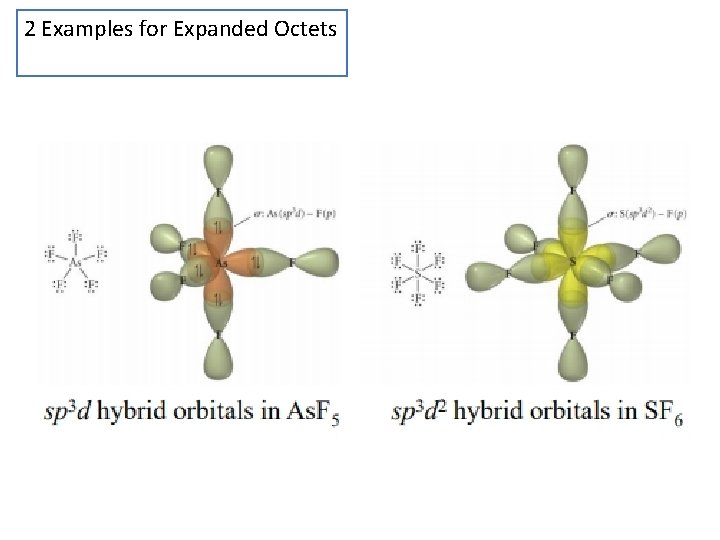
2 Examples for Expanded Octets

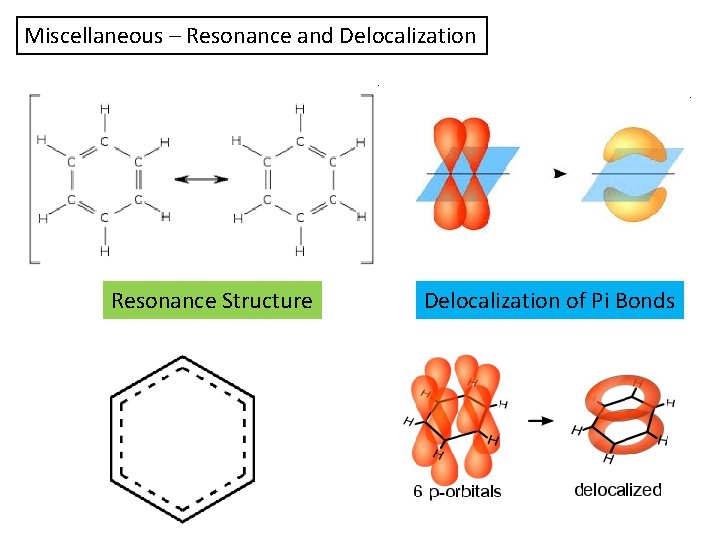
Miscellaneous – Resonance and Delocalization Resonance Structure Delocalization of Pi Bonds

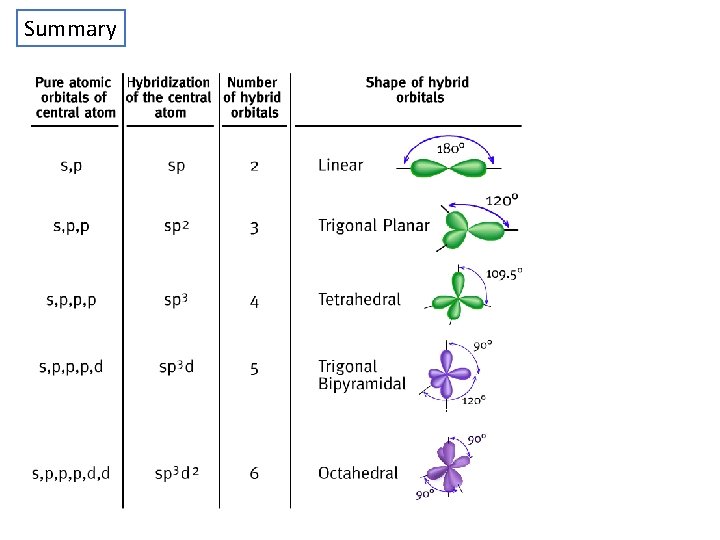
Summary


