Charles Law Temperature Changes Matter Solids Liquids expand












- Slides: 23

Charles’ Law

Temperature Changes & Matter Solids & Liquids expand contract as temperature changes. – Change is usually v. small. Gases show large volume changes with temperature changes.

Jacques Charles Balloonist. 1787 did expts on how volume of gases depends on temperature.

How do hot air balloons work?

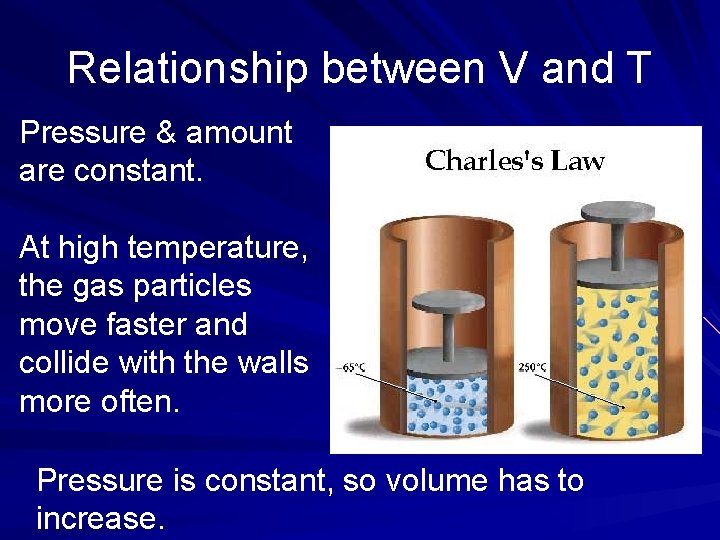
Relationship between V and T Pressure & amount are constant. At high temperature, the gas particles move faster and collide with the walls more often. Pressure is constant, so volume has to increase.

Charles’ Law Tiger Graphic

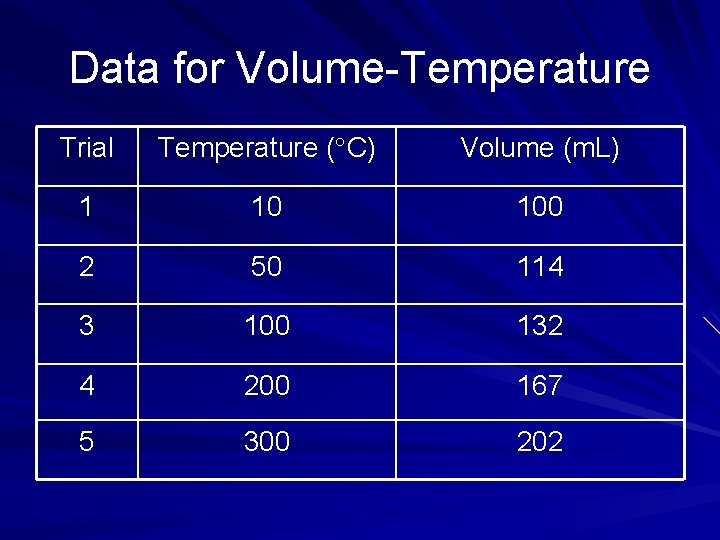
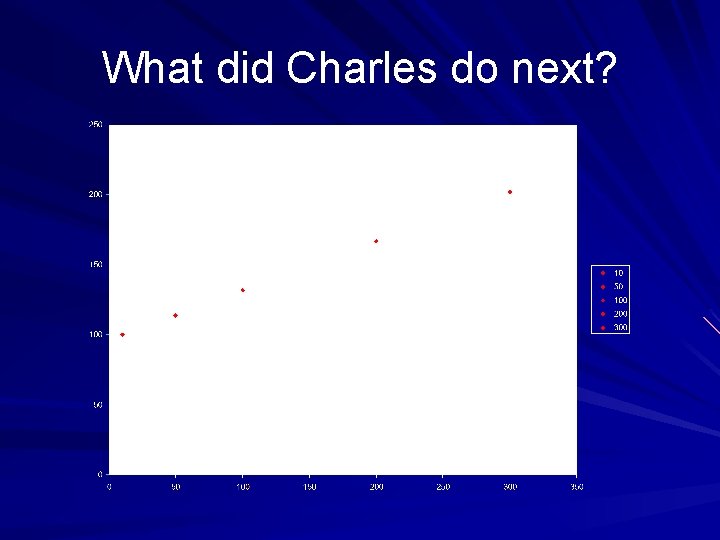
Data for Volume-Temperature Trial Temperature ( C) Volume (m. L) 1 10 100 2 50 114 3 100 132 4 200 167 5 300 202

What did Charles do next?

Linear Relationship Plot Volume vs. C and you get a straight line. The relationship between Volume and C is linear. The equation of a line is: Y = m. X + b.

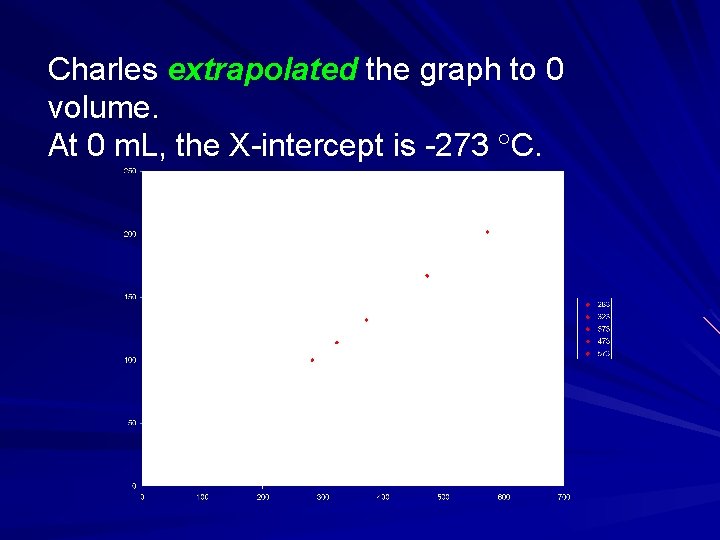
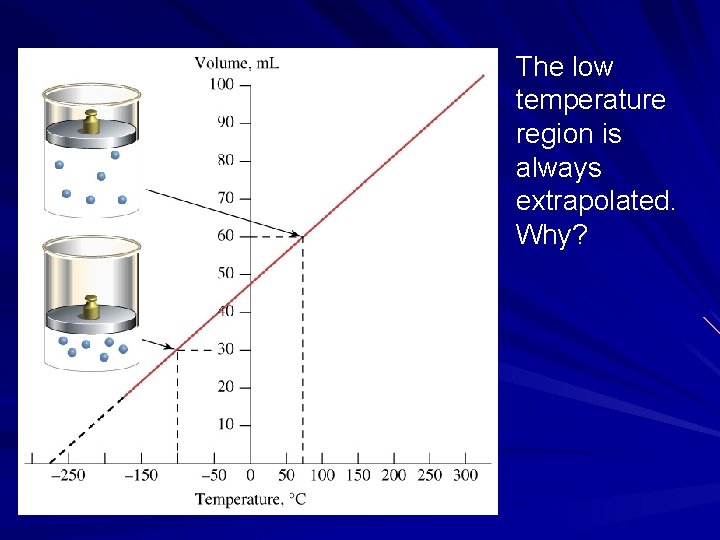
Charles extrapolated the graph to 0 volume. At 0 m. L, the X-intercept is -273 C.

Hints of Kelvin scale Charles extrapolated his data to see the temperature at which the volume was 0. 1 st indication that the temperature -273 C might have a fundamental meaning. Why did Charles have to extrapolate his lines in this temperature range instead of taking data?

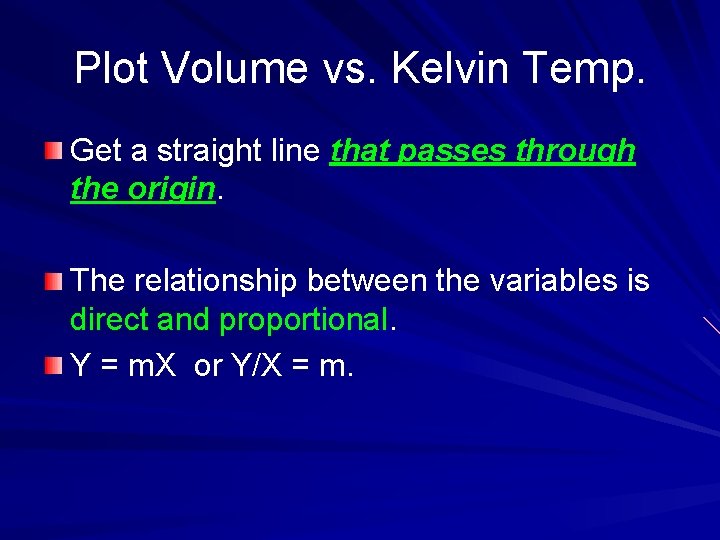
Plot Volume vs. Kelvin Temp. Get a straight line that passes through the origin. The relationship between the variables is direct and proportional. Y = m. X or Y/X = m.

Charles’ Law: Verbal The volume of a gas at constant pressure varies directly with its absolute (Kelvin) temperature.

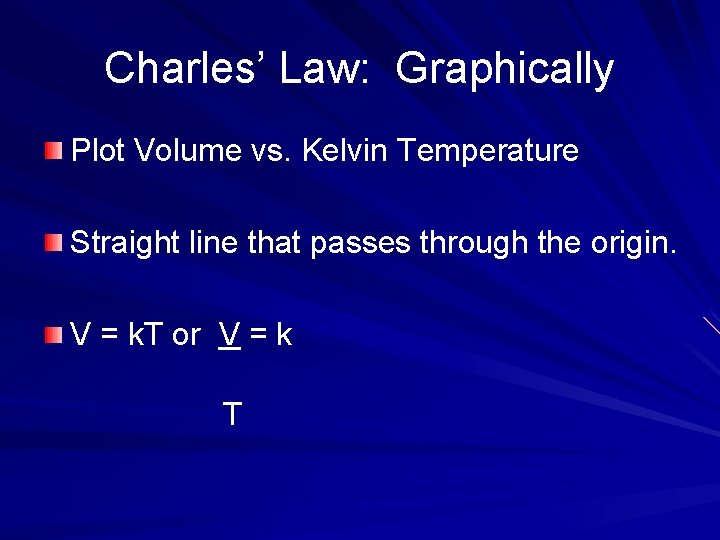
Charles’ Law: Graphically Plot Volume vs. Kelvin Temperature Straight line that passes through the origin. V = k. T or V = k T

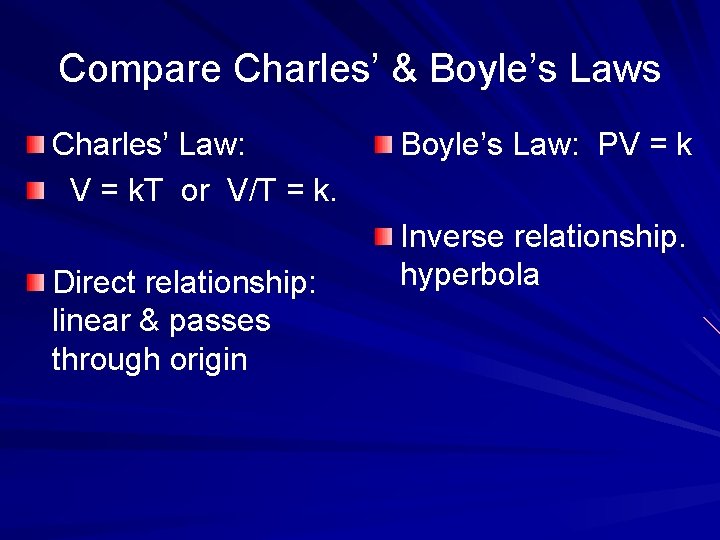
Compare Charles’ & Boyle’s Laws Charles’ Law: V = k. T or V/T = k. Direct relationship: linear & passes through origin Boyle’s Law: PV = k Inverse relationship. hyperbola

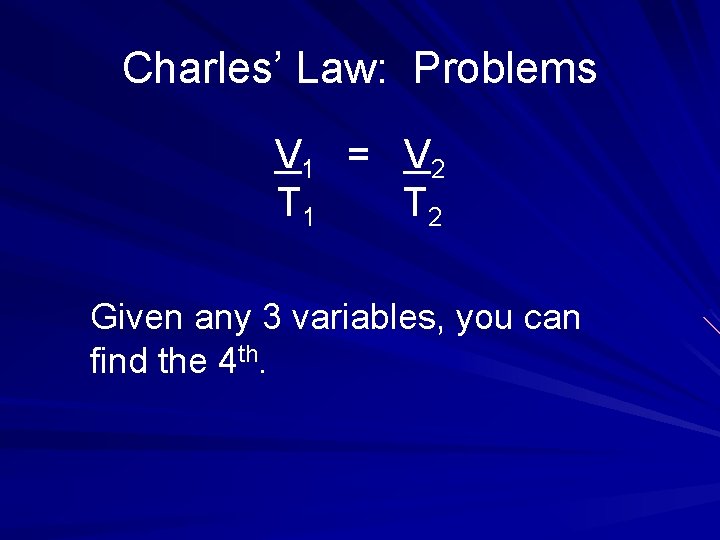
Charles’ Law: Problems V 1 = V 2 T 1 T 2 Given any 3 variables, you can find the 4 th.

The low temperature region is always extrapolated. Why?

Gay-Lussac’s Law

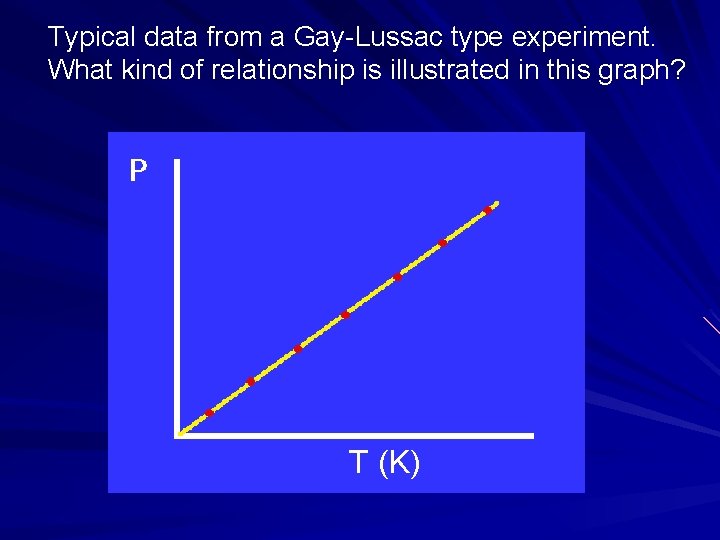
Typical data from a Gay-Lussac type experiment. What kind of relationship is illustrated in this graph? T (K)

Gay-Lussac’s Law Describes relationship between pressure & temperature at constant volume & amount. P = k. T or P/T = k What kind of relationship is this? Straight line with y-intercept of zero. It’s direct.

Gay-Lussac: Verbal The pressure exerted by a confined gas is directly related to the Kelvin temperature at constant volume.

Gay-Lussac’s Law P 1 = P 2 T 1 T 2 Useful problem-solving format.

Gay-Lussac Examined relationship betw P & T at constant V and moles If T , the gas molecules will move faster, colliding with the walls more frequently & more energetically. Pressure results from collisions between gas molecules & walls of container. So, if T , we expect P .