Charging Things Up 4 1 1 Elementary Charge


- Slides: 12

Charging Things Up 4. 1. 1 Elementary Charge Created by Mr. Wilson Modified by Mr. Wanninkhof

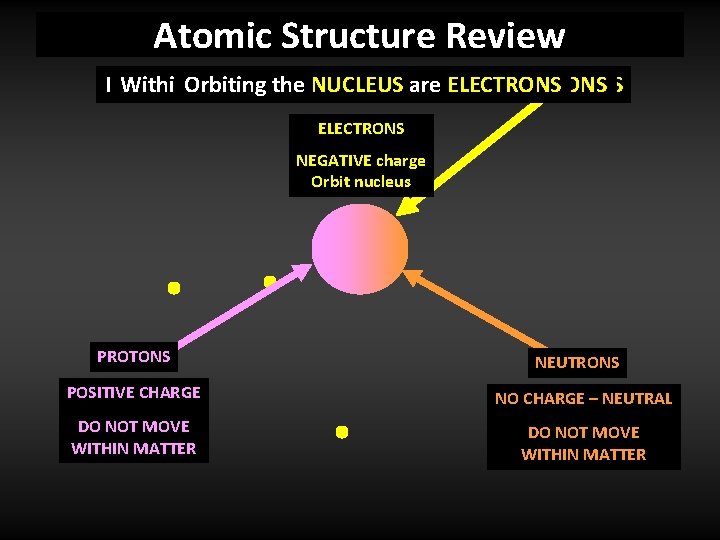
Atomic Structure Review Matter Within Atis. Orbiting the made center NUCLEUS up theof of. NUCLEUS small each are two atom structures are types is. ELECTRONS a NUCLEUS ofcalled NUCLEONS ATOMS ELECTRONS NEGATIVE charge Orbit nucleus PROTONS NEUTRONS POSITIVE CHARGE NO CHARGE – NEUTRAL DO NOT MOVE WITHIN MATTER

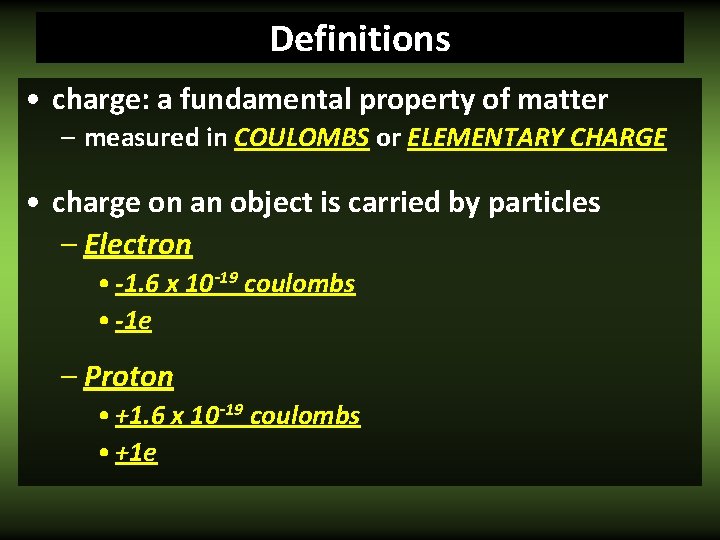
Definitions • charge: a fundamental property of matter – measured in COULOMBS or ELEMENTARY CHARGE • charge on an object is carried by particles – Electron • -1. 6 x 10 -19 coulombs • -1 e – Proton • +1. 6 x 10 -19 coulombs • +1 e

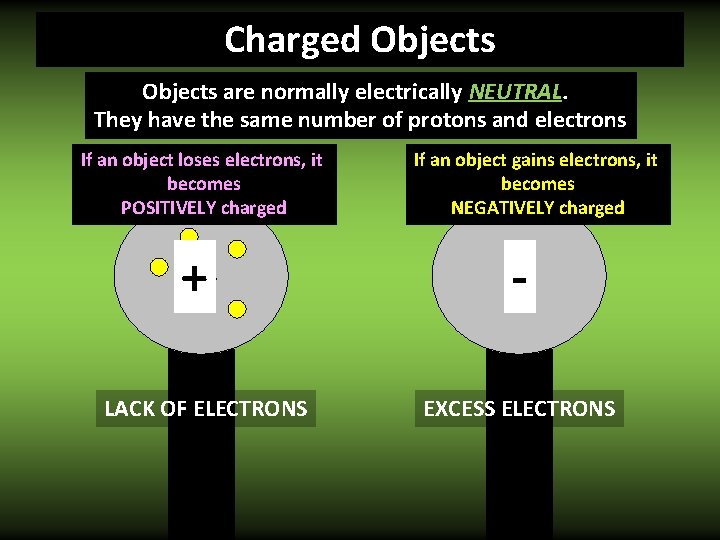
Charged Objects are normally electrically NEUTRAL. They have the same number of protons and electrons If an object loses electrons, it becomes POSITIVELY charged + LACK OF ELECTRONS If an object gains electrons, it becomes NEGATIVELY charged EXCESS ELECTRONS

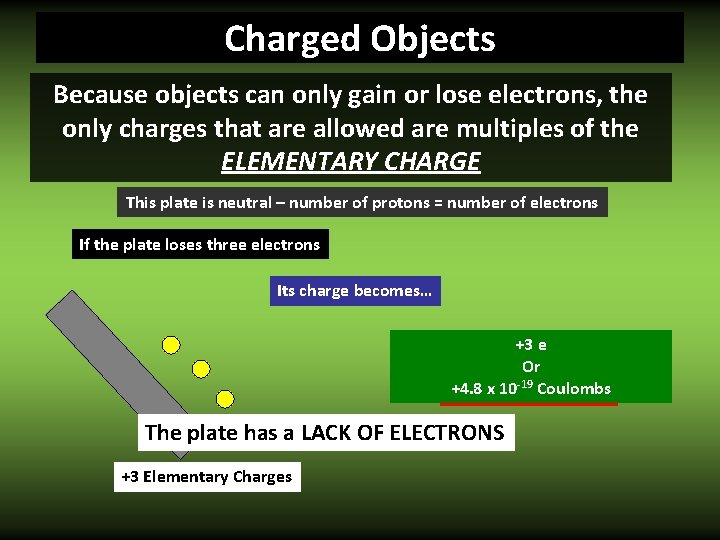
Charged Objects Because objects can only gain or lose electrons, the only charges that are allowed are multiples of the ELEMENTARY CHARGE This plate is neutral – number of protons = number of electrons If the plate loses three one electron two electrons Its charge becomes… +3 +2 +1 ee Or -19 Coulombs +3. 2 x 10 -19 +4. 8 +1. 6 The plate has a LACK OF ELECTRONS +1 Elementary Charges +2 +3 Charge

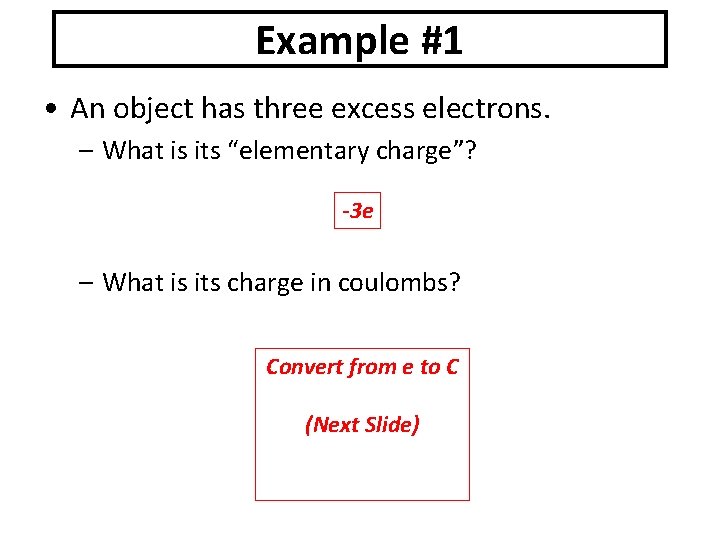
Example #1 • An object has three excess electrons. – What is its “elementary charge”? -3 e – What is its charge in coulombs? Convert from e to C (Next Slide)

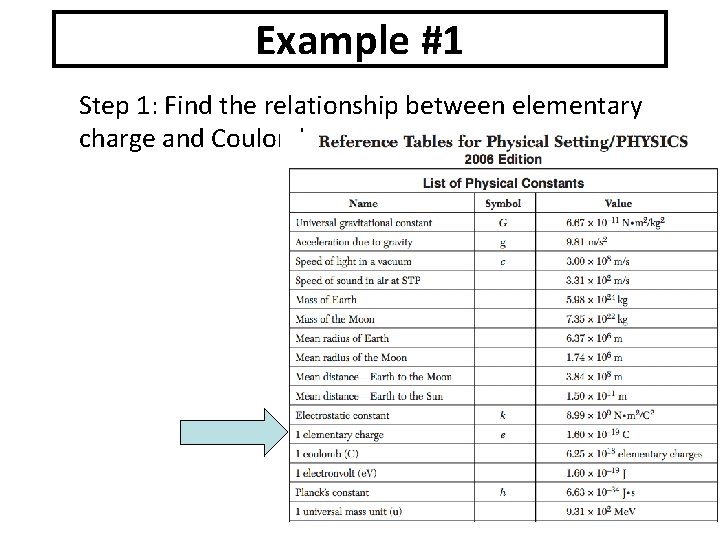
Example #1 Step 1: Find the relationship between elementary charge and Coulombs

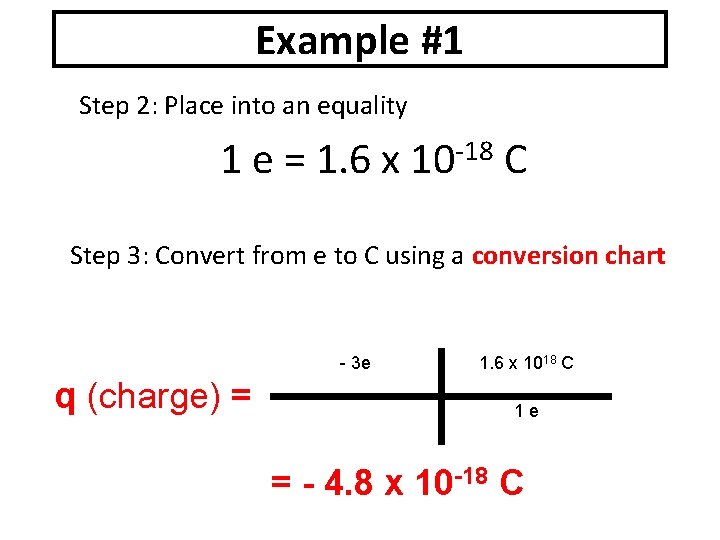
Example #1 Step 2: Place into an equality 1 e = 1. 6 x -18 10 C Step 3: Convert from e to C using a conversion chart - 3 e q (charge) = 1. 6 x 1018 C 1 e = - 4. 8 x 10 -18 C

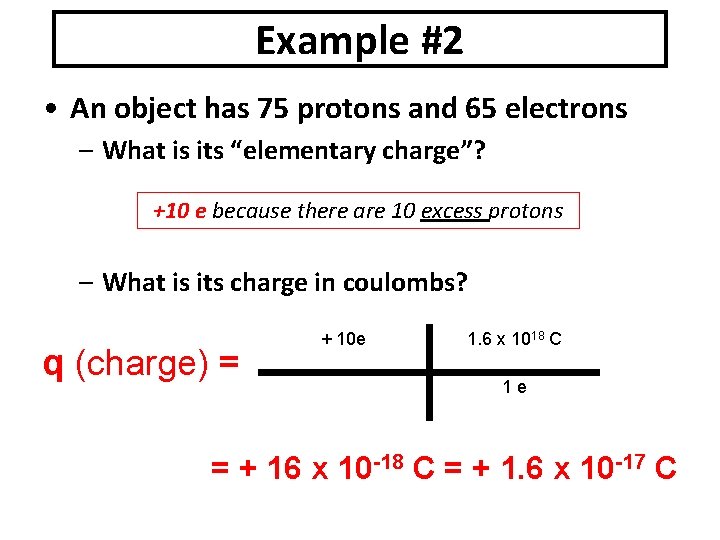
Example #2 • An object has 75 protons and 65 electrons – What is its “elementary charge”? +10 e because there are 10 excess protons – What is its charge in coulombs? q (charge) = + 10 e 1. 6 x 1018 C 1 e = + 16 x 10 -18 C = + 1. 6 x 10 -17 C

Law of Conservation of Charge • The total amount of charge in a closed system remains constant – charge is not created or destroyed, it only moves from one object to another • Charge “moves” as a result of ELECTRON movement ONLY!!!

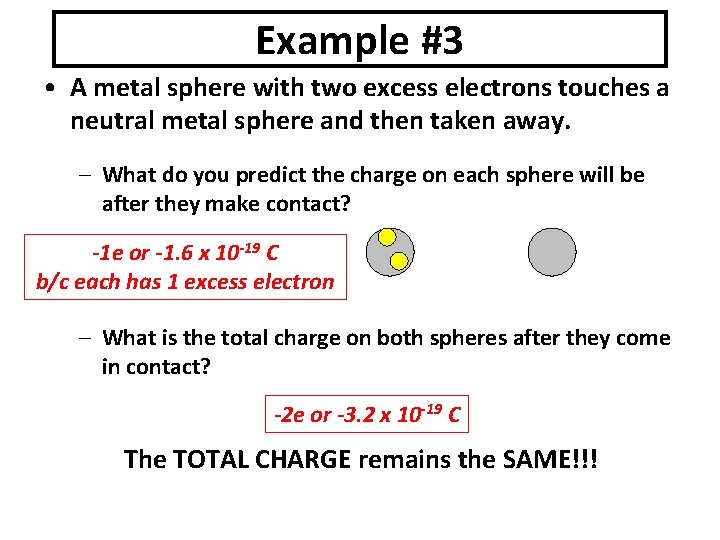
Example #3 • A metal sphere with two excess electrons touches a neutral metal sphere and then taken away. – What do you predict the charge on each sphere will be after they make contact? -1 e or -1. 6 x 10 -19 C b/c each has 1 excess electron – What is the total charge on both spheres after they come in contact? -2 e or -3. 2 x 10 -19 C The TOTAL CHARGE remains the SAME!!!

End of 5. 1. 1 - PRACTICE