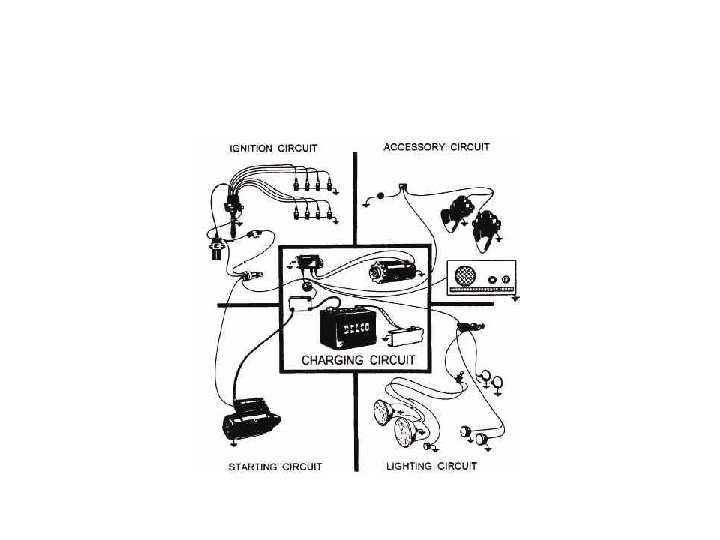
CHARGING CIRCUIT Lead Acid Battery Functions of charging

CHARGING CIRCUIT Lead Acid Battery
Functions of charging system • It recharges the battery after engine cranking or after the use of electrical accessories • It supplies all the electricity for the vehicle when the engine is running. • It must change output to meet different electrical loads. • It provides a voltage output that is slightly higher than battery voltage.
STORAGE BATTERY • The storage battery is the heart of the charging circuit • It is an electrochemical device for producing and storing electricity. Functions: • It must operate the starting motor, ignition system, electronic fuel injection system, and other electrical devices for the engine during engine cranking and starting. • It must supply ALL of the electrical power for the vehicle when the engine is not running. • It must help the charging system provide electricity when current demands are above the output limit of the charging system.

• It must act as a capacitor (voltage stabilizer) that smoothes current flow through the electrical system. • It must store energy (electricity) for extended periods.

Lead-acid cell-type battery. • This type of battery produces direct current (dc) electricity that flows in only one direction. • When the battery is discharging (current • flowing out of the battery), it changes chemical energy into electrical energy, thereby, releasing stored energy. • During charging (current flowing into the battery from the charging system), electrical energy is converted into chemical energy. • The battery can then store energy until the vehicle requires it.
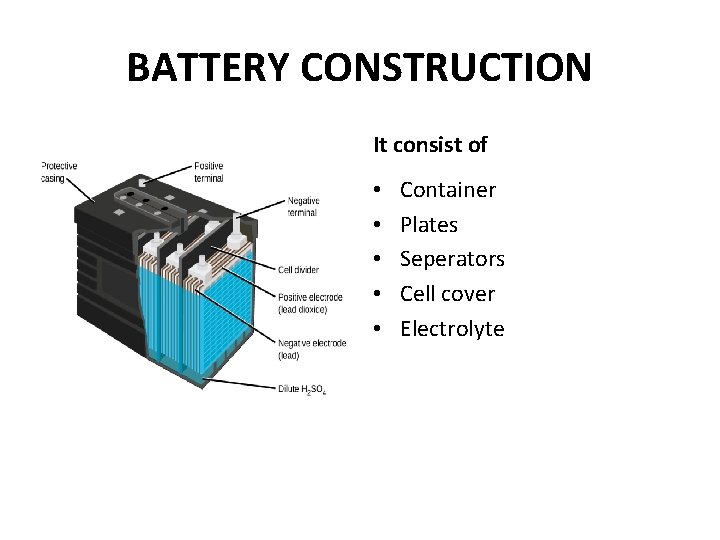
BATTERY CONSTRUCTION It consist of • • • Container Plates Seperators Cell cover Electrolyte

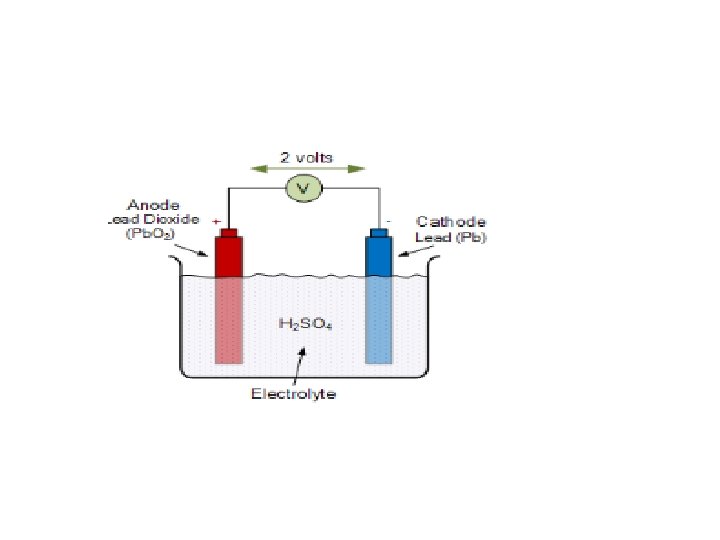
Discharing process • The lead acid storage battery is formed by dipping lead peroxide plate and sponge lead plate in dilute sulfuric acid. • A load is connected externally between these plates. In diluted sulfuric acid the molecules of the acid split into positive hydrogen ions (H+) and negative sulfate ions (SO 4 − −). • The hydrogen ions when reach at Pb. O 2 plate, they receive electrons from it and become hydrogen atom which again attack Pb. O 2 and form Pb. O and H 2 O (water). • This Pb. O reacts with H 2 SO 4 and forms Pb. SO 4 and H 2 O (water). • SO 4 − − ions are moving freely in the solution so some of them will reach to pure Pb plate where they give their extra electrons and become radical SO 4. As the radical SO 4 cannot exist alone it will attack Pb and will form Pb. SO 4. As H+ ions take electrons from Pb. O 2 plate and SO 4 − − ions give electrons to Pb plate, there would be an inequality of electrons between these two plates. Hence there would be a flow of current through the external load between these plates for balancing this inequality of electrons. This process is called discharging of lead acid battery.
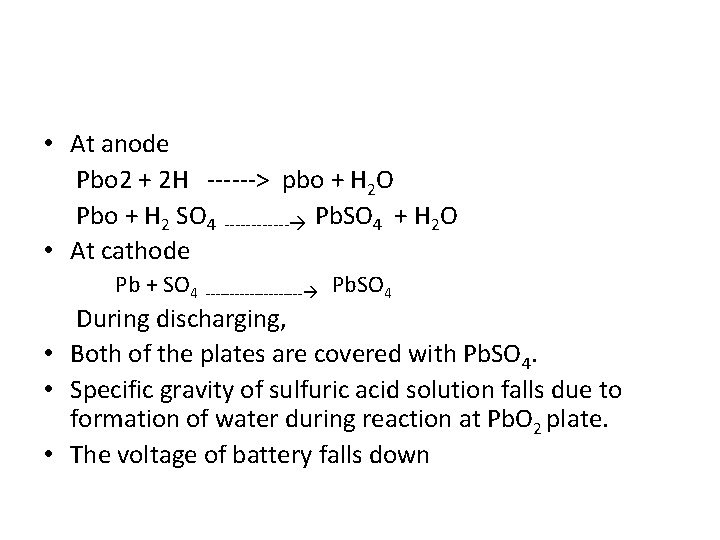
• At anode Pbo 2 + 2 H ------> pbo + H 2 O Pbo + H 2 SO 4 ------ Pb. SO 4 + H 2 O • At cathode Pb + SO 4 ---------- Pb. SO 4 During discharging, • Both of the plates are covered with Pb. SO 4. • Specific gravity of sulfuric acid solution falls due to formation of water during reaction at Pb. O 2 plate. • The voltage of battery falls down
Charging process Now we will disconnect the load and connect Pb. SO 4 covered Pb. O 2 plate with positive terminal of an external DC source and Pb. O 2 covered Pb plate with negative terminal of that DC source. During discharging, the density of sulfuric acid falls but there still sulfuric acid exists in the solution. This sulfuric acid also remains as H+ and SO 4 − − ions in the solution. Hydrogen ions being positively charged, move to the electrode (cathode) connected with negative terminal of the DC source. Here each H+ ion takes one electron from that and becomes hydrogen atom. These hydrogen atoms then attack Pb. SO 4 and form lead and sulfuric acid. SO 4 − − ions (anions) move towards the electrode (anode) connected with positive terminal of DC source where they will give up their extra electrons and become radical SO 4. This radical SO 4 cannot exist alone hence reacts with Pb. SO 4 of anode and forms lead peroxide (Pb. O 2) and sulfuric acid (H 2 SO 4).
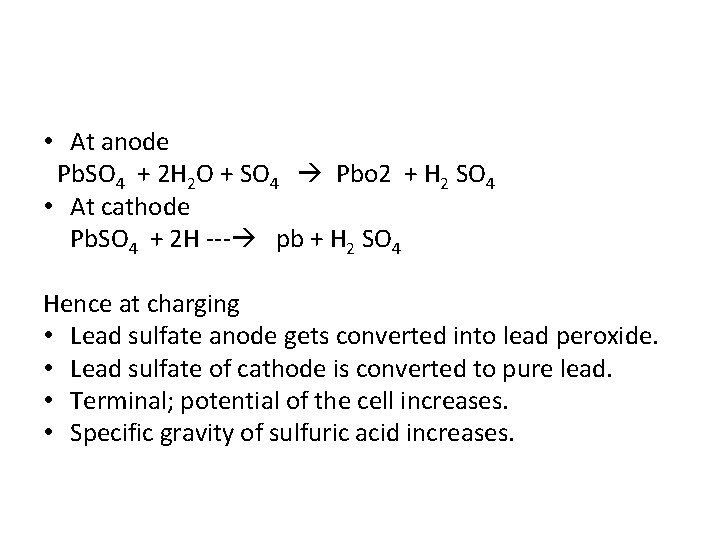
• At anode Pb. SO 4 + 2 H 2 O + SO 4 Pbo 2 + H 2 SO 4 • At cathode Pb. SO 4 + 2 H --- pb + H 2 SO 4 Hence at charging • Lead sulfate anode gets converted into lead peroxide. • Lead sulfate of cathode is converted to pure lead. • Terminal; potential of the cell increases. • Specific gravity of sulfuric acid increases.

- Slides: 14