CHARGES HOW DO YOU GET CHARGES ON AN


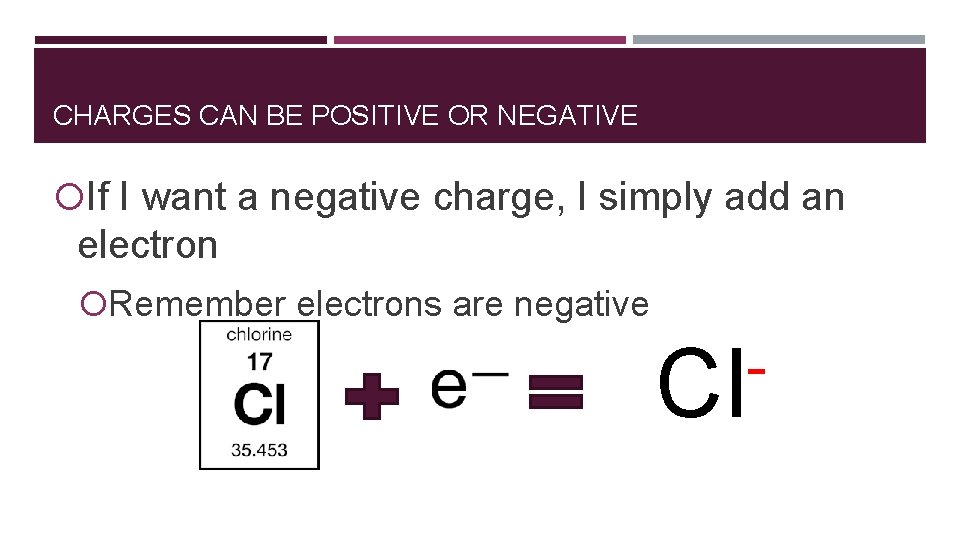



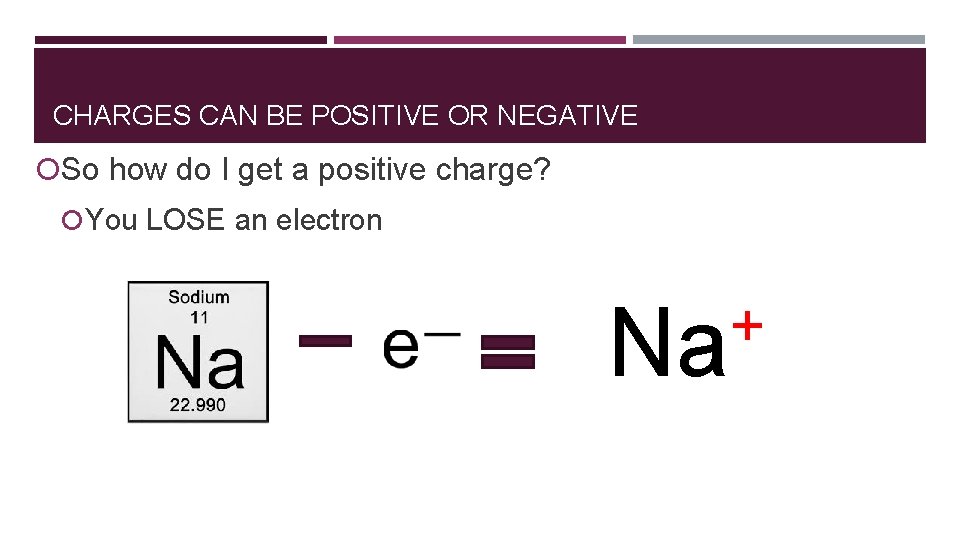

- Slides: 21

CHARGES HOW DO YOU GET CHARGES ON AN ELEMENT?

ION An ion is an element with a charge Na+ Cl-
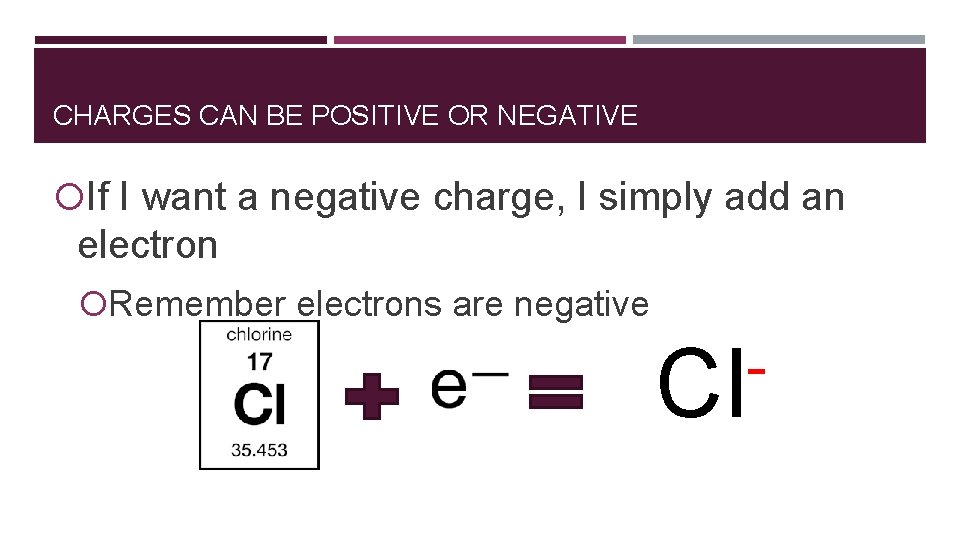
CHARGES CAN BE POSITIVE OR NEGATIVE If I want a negative charge, I simply add an electron Remember electrons are negative Cl

CHARGES CAN BE POSITIVE OR NEGATIVE If I want a positive charge can I add a proton?

CHARGES CAN BE POSITIVE OR NEGATIVE If I want a positive charge can I add a proton?

CHARGES CAN BE POSITIVE OR NEGATIVE You can never ever never add a proton. Why? What happens if I add a proton? You change the element. Protons are in the nucleus and we do not want to mess with that

CHARGES CAN BE POSITIVE OR NEGATIVE So how do I get a positive charge? You LOSE an electron
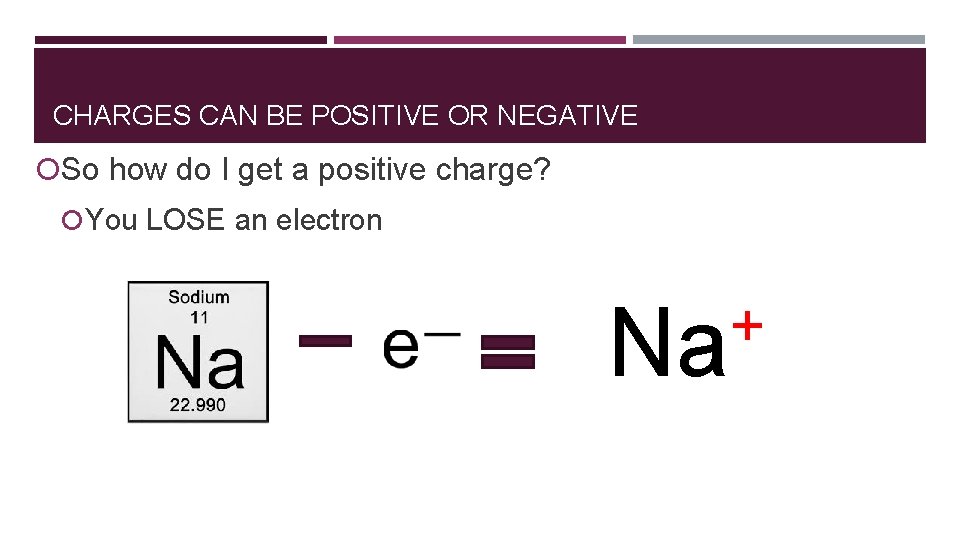
CHARGES CAN BE POSITIVE OR NEGATIVE So how do I get a positive charge? You LOSE an electron + Na

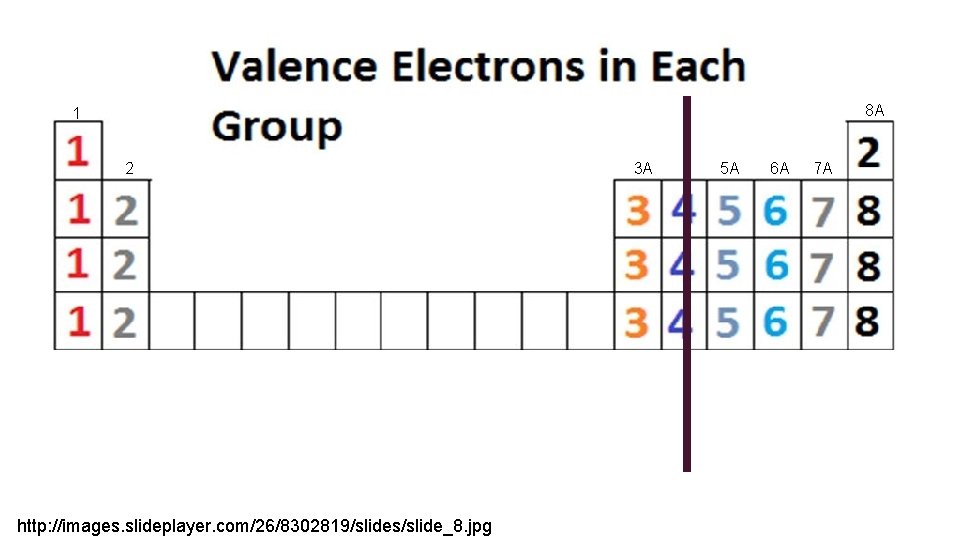
SO HOW DO I KNOW IF I WILL GAIN OR LOSE AN ELECTRON? Every element wants to have a full outer shell. Some times it’s easier to lose an electron rather than gain many. Sometimes it’s easier to gain a few electrons rather than lose many. It’s all about that outer shell (valence electrons)

8 A 1 2 http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 3 A 5 A 6 A 7 A

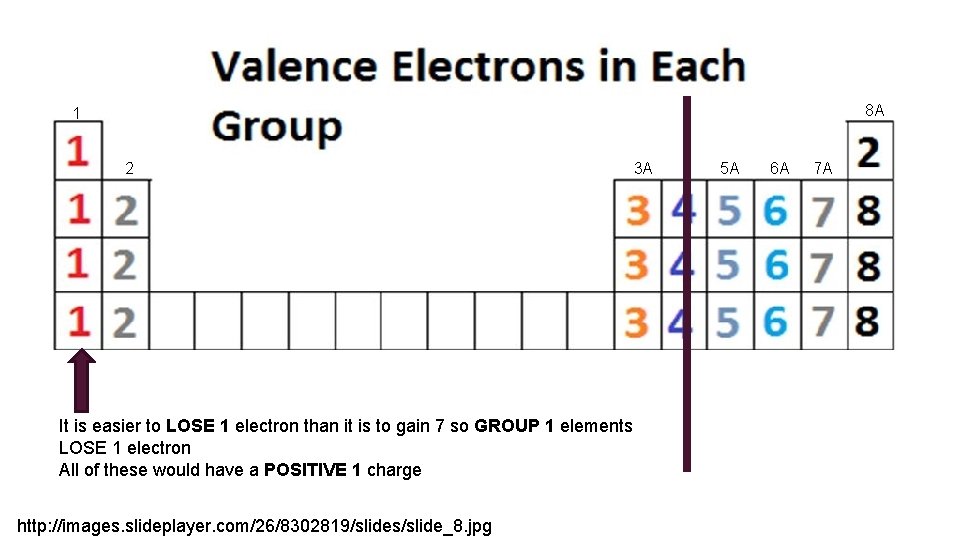
8 A 1 2 It is easier to LOSE 1 electron than it is to gain 7 so GROUP 1 elements LOSE 1 electron All of these would have a POSITIVE 1 charge http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 3 A 5 A 6 A 7 A

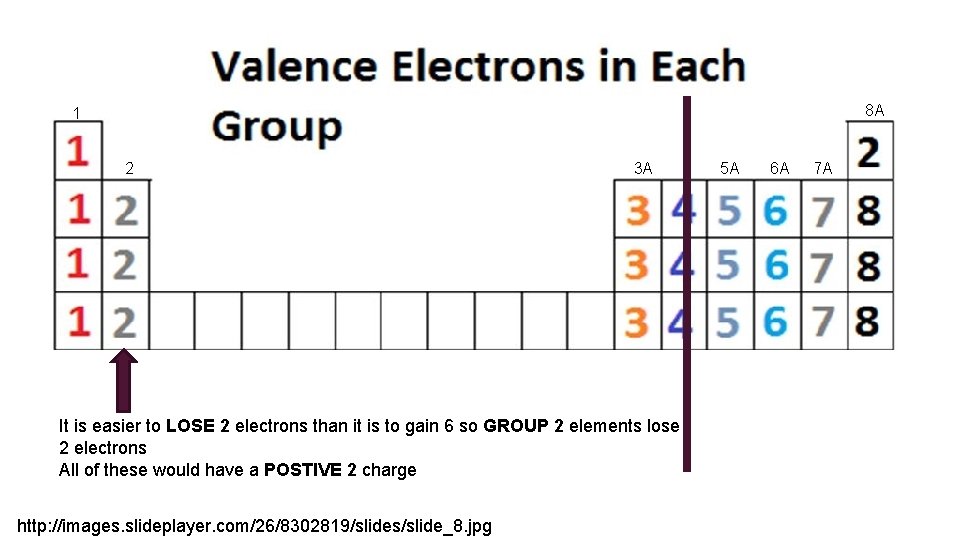
8 A 1 2 3 A It is easier to LOSE 2 electrons than it is to gain 6 so GROUP 2 elements lose 2 electrons All of these would have a POSTIVE 2 charge http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A

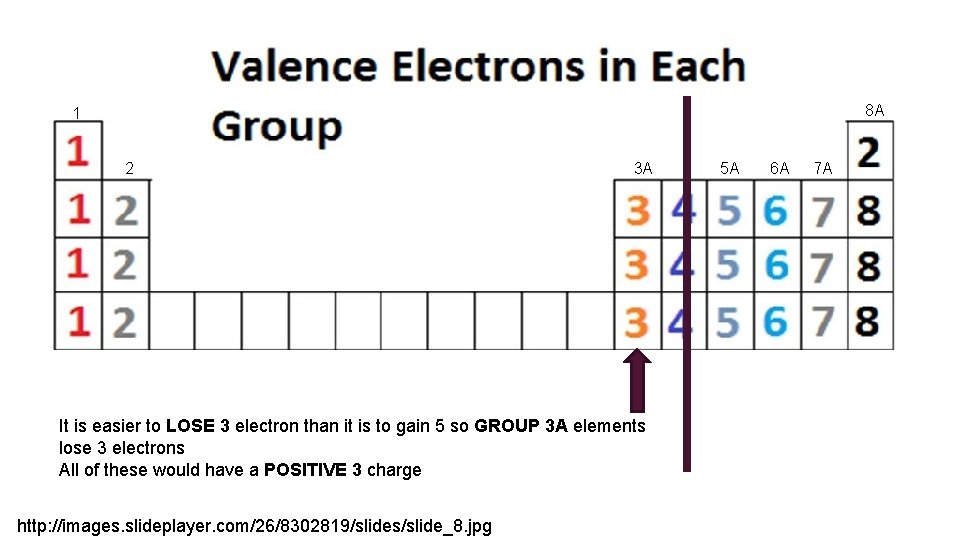
8 A 1 2 3 A It is easier to LOSE 3 electron than it is to gain 5 so GROUP 3 A elements lose 3 electrons All of these would have a POSITIVE 3 charge http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A

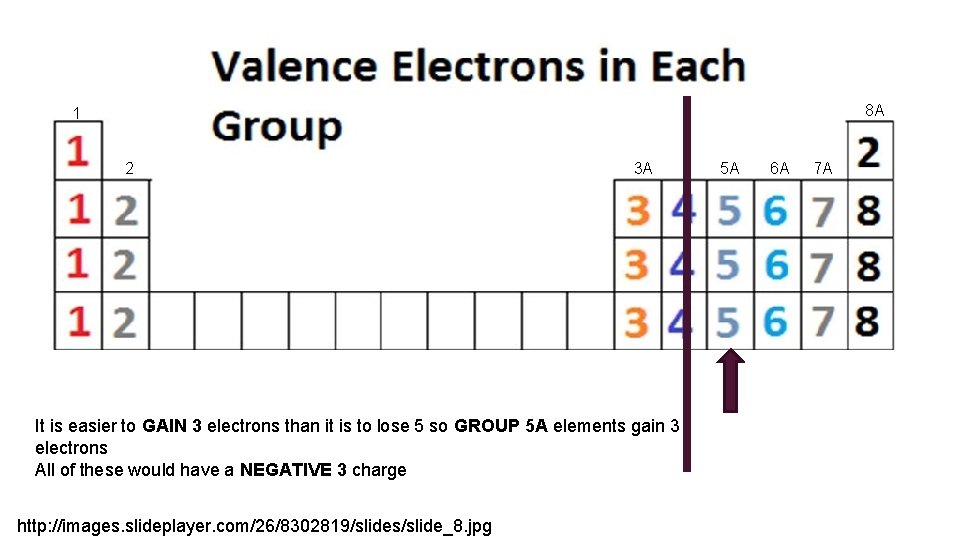
8 A 1 2 3 A It is easier to GAIN 3 electrons than it is to lose 5 so GROUP 5 A elements gain 3 electrons All of these would have a NEGATIVE 3 charge http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A

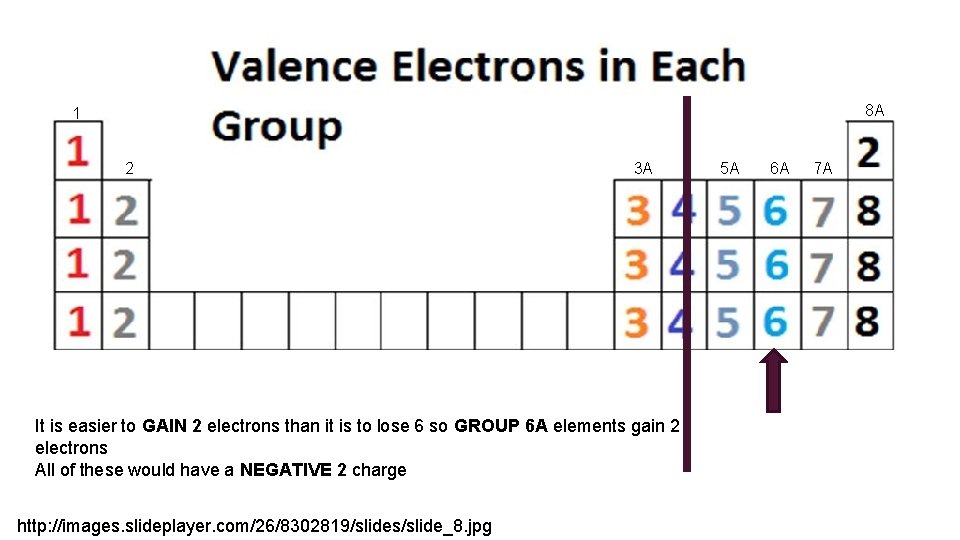
8 A 1 2 3 A It is easier to GAIN 2 electrons than it is to lose 6 so GROUP 6 A elements gain 2 electrons All of these would have a NEGATIVE 2 charge http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A

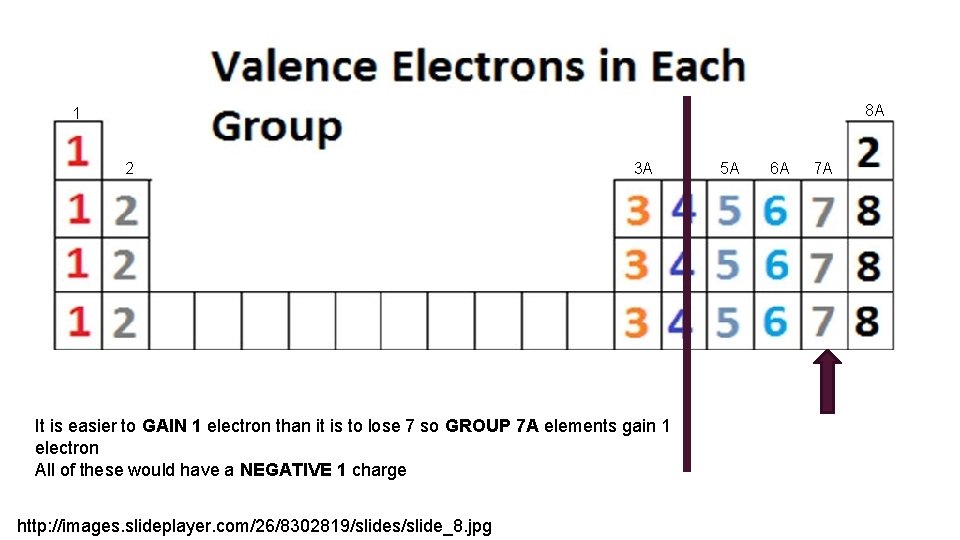
8 A 1 2 3 A It is easier to GAIN 1 electron than it is to lose 7 so GROUP 7 A elements gain 1 electron All of these would have a NEGATIVE 1 charge http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A

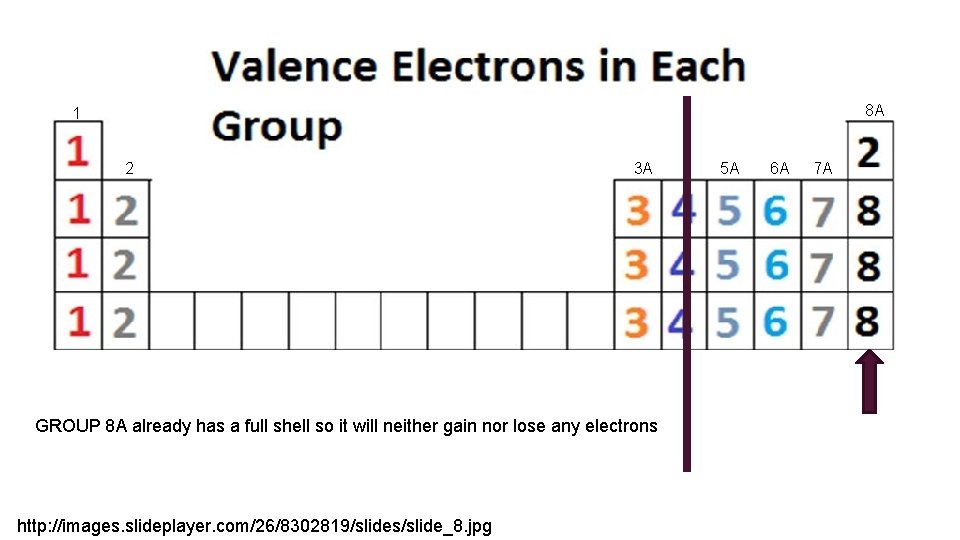
8 A 1 2 3 A GROUP 8 A already has a full shell so it will neither gain nor lose any electrons http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A

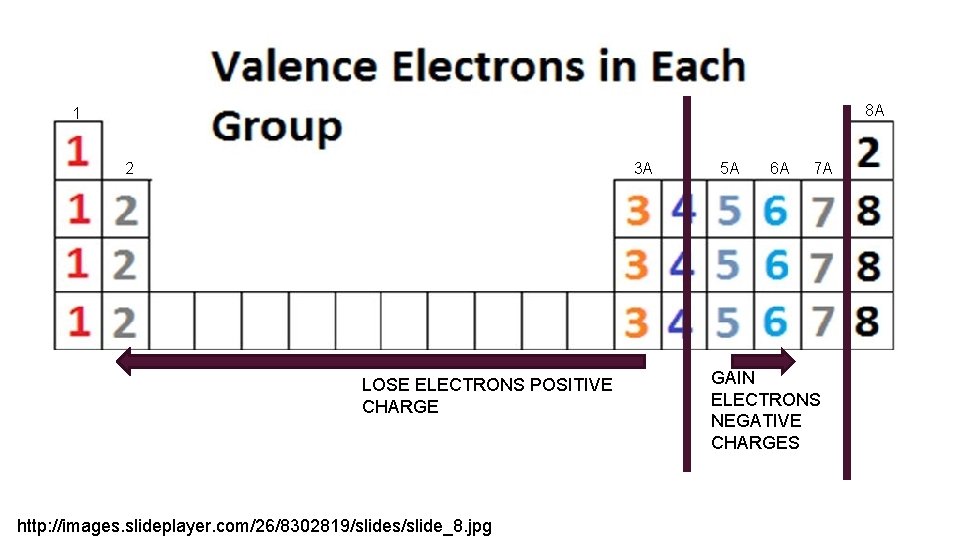
8 A 1 2 3 A LOSE ELECTRONS POSITIVE CHARGE http: //images. slideplayer. com/26/8302819/slides/slide_8. jpg 5 A 6 A 7 A GAIN ELECTRONS NEGATIVE CHARGES

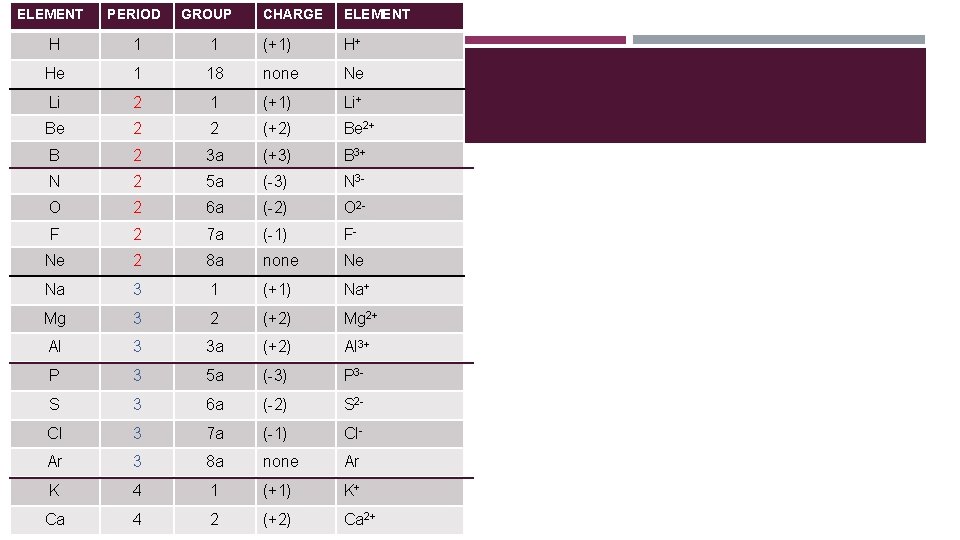
ELEMENT PERIOD GROUP CHARGE ELEMENT H 1 1 (+1) H+ He 1 18 none Ne Li 2 1 (+1) Li+ ELECTRON CONFIGURATION 2 2 (+2) Be Be 2+ B 2 3 a (+3) B 3+ N 2 5 a (-3) N 3 - O 2 6 a (-2) O 2 - F 2 7 a (-1) F- Ne 2 8 a none Ne Na 3 1 (+1) Na+ Mg 3 2 (+2) Mg 2+ Al 3 3 a (+2) Al 3+ P 3 5 a (-3) P 3 - S 3 6 a (-2) S 2 - Cl 3 7 a (-1) Cl- Ar 3 8 a none Ar K 4 1 (+1) K+ Ca 4 2 (+2) Ca 2+

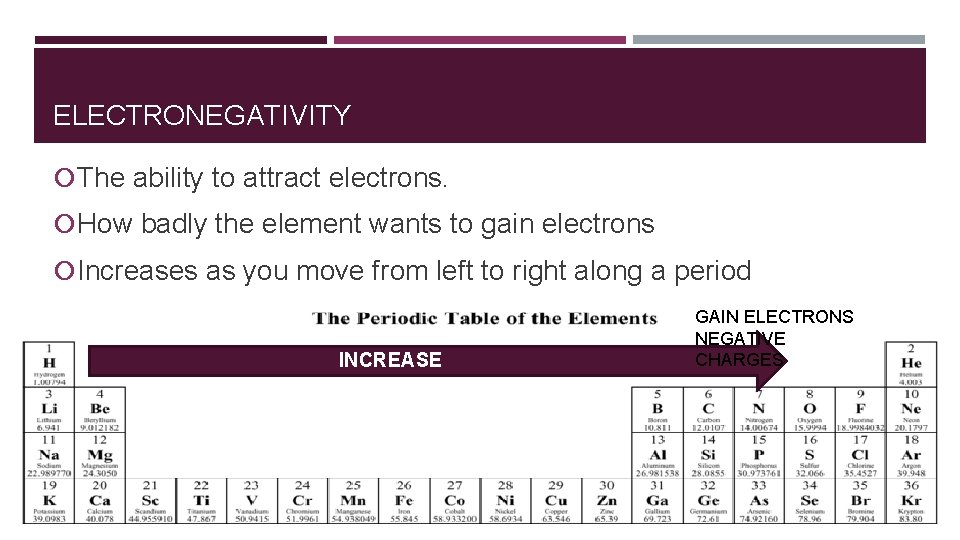
ELECTRONEGATIVITY The ability to attract electrons. How badly the element wants to gain electrons Increases as you move from left to right along a period INCREASE GAIN ELECTRONS NEGATIVE CHARGES

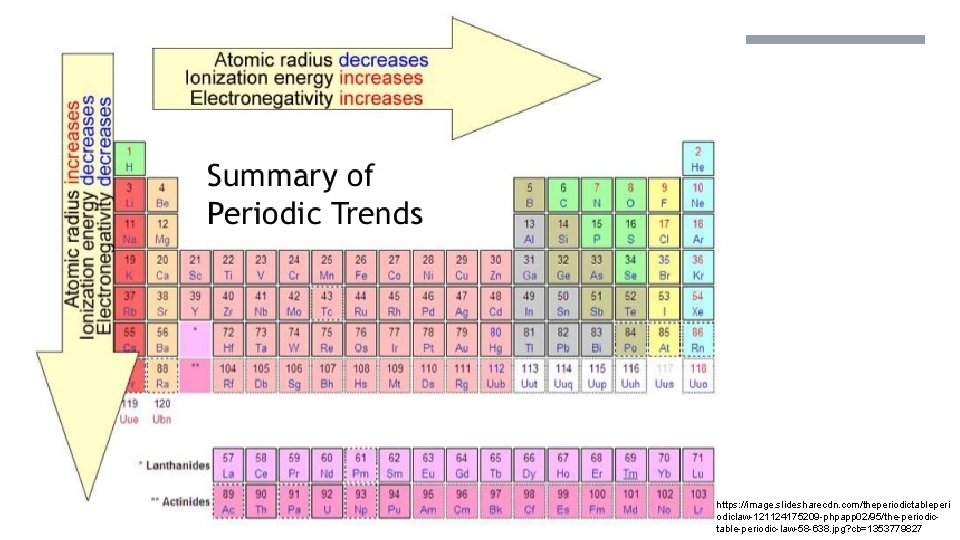
https: //image. slidesharecdn. com/theperiodictableperi odiclaw-121124175209 -phpapp 02/95/the-periodictable-periodic-law-58 -638. jpg? cb=1353779827