Characterization Techniques Gas Adsorption Study of the porosity



- Slides: 32

Characterization Techniques Gas Adsorption: Study of the porosity of materials Bartłomiej Gaweł, Norwegian University of Science and Technology (NTNU) 17. 04. 2015

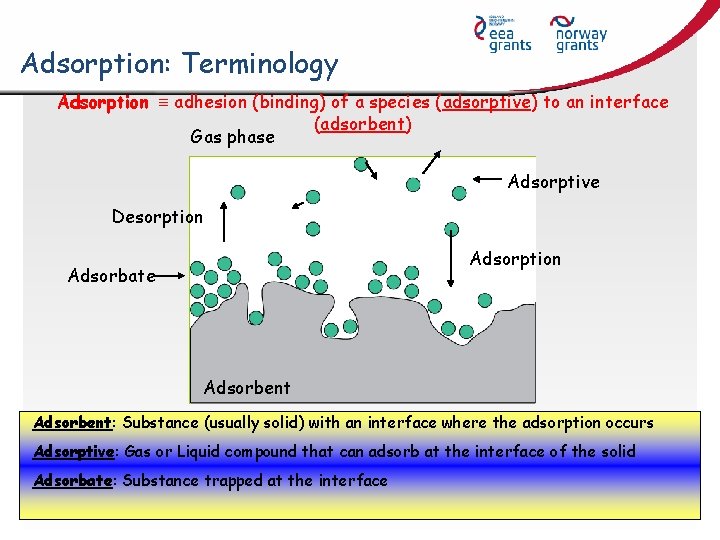
Adsorption: Terminology Adsorption adhesion (binding) of a species (adsorptive) to an interface (adsorbent) Gas phase Adsorptive Desorption Adsorbate Adsorbent: Substance (usually solid) with an interface where the adsorption occurs Adsorptive: Gas or Liquid compound that can adsorb at the interface of the solid Adsorbate: Substance trapped at the interface

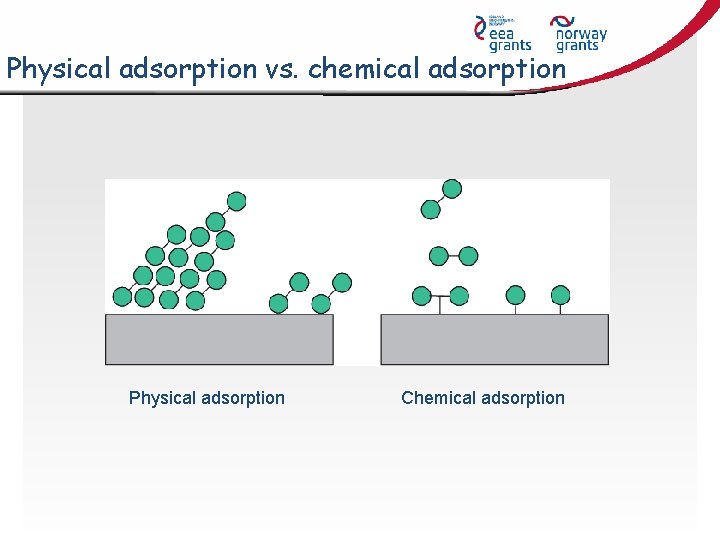
Physical adsorption vs. chemical adsorption Physical adsorption Chemical adsorption

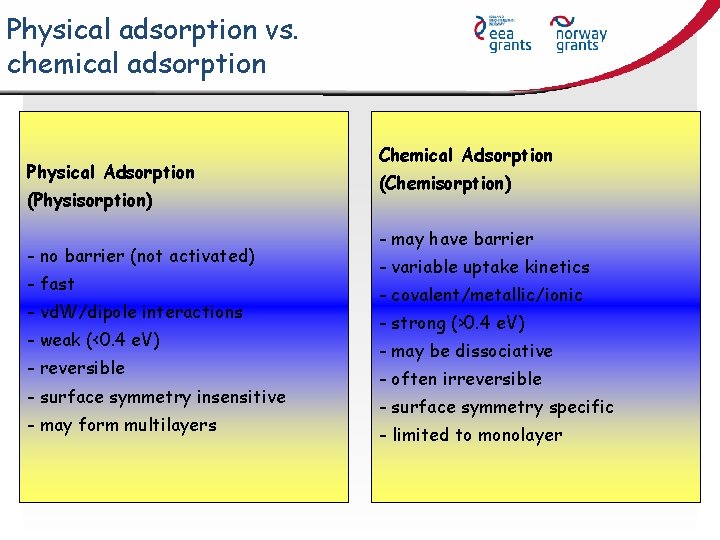
Physical adsorption vs. chemical adsorption Physical Adsorption (Physisorption) - no barrier (not activated) - fast - vd. W/dipole interactions - weak (<0. 4 e. V) - reversible - surface symmetry insensitive - may form multilayers Chemical Adsorption (Chemisorption) - may have barrier - variable uptake kinetics - covalent/metallic/ionic - strong (>0. 4 e. V) - may be dissociative - often irreversible - surface symmetry specific - limited to monolayer

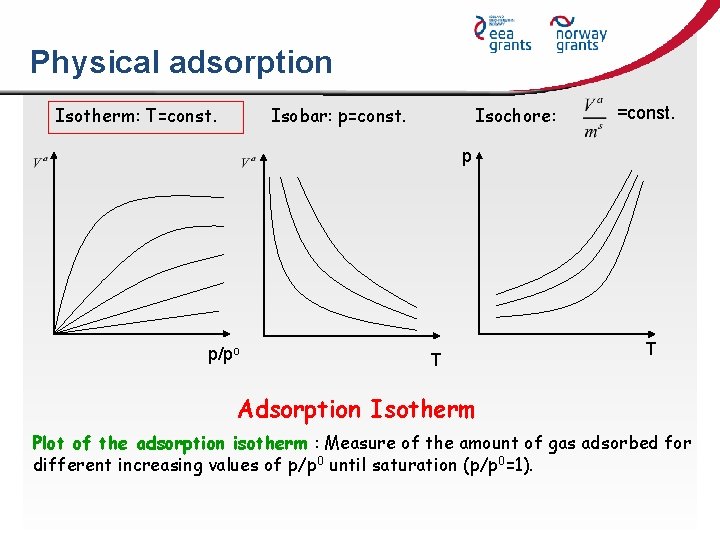
Physical adsorption Isotherm: T=const. Isobar: p=const. Isochore: =const. p p/po T T Adsorption Isotherm Plot of the adsorption isotherm : Measure of the amount of gas adsorbed for different increasing values of p/p 0 until saturation (p/p 0=1).

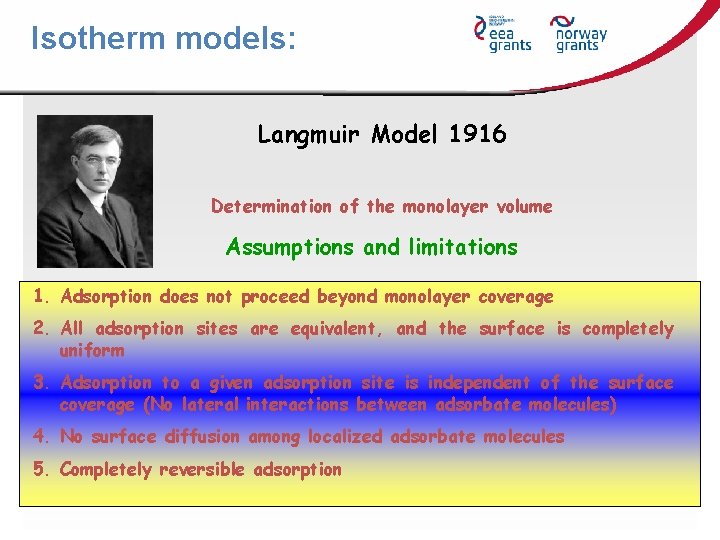
Isotherm models: Langmuir Model 1916 Determination of the monolayer volume Assumptions and limitations 1. Adsorption does not proceed beyond monolayer coverage 2. All adsorption sites are equivalent, and the surface is completely uniform 3. Adsorption to a given adsorption site is independent of the surface coverage (No lateral interactions between adsorbate molecules) 4. No surface diffusion among localized adsorbate molecules 5. Completely reversible adsorption

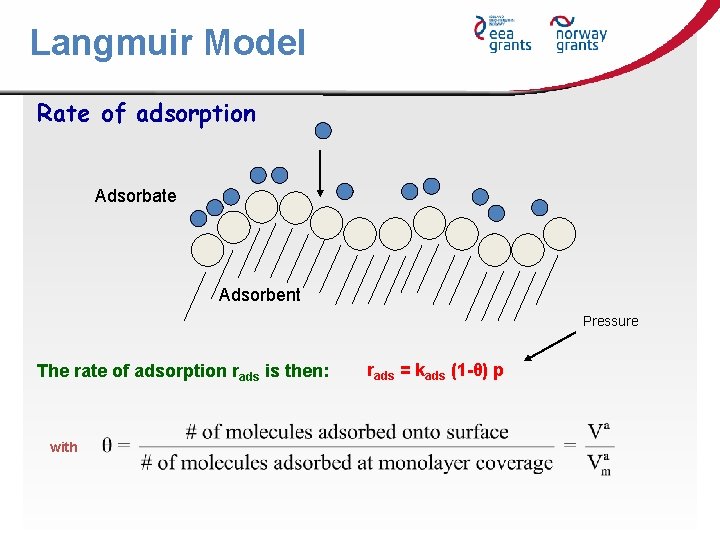
Langmuir Model Rate of adsorption Adsorbate Adsorbent Pressure The rate of adsorption rads is then: with rads = kads (1 -θ) p

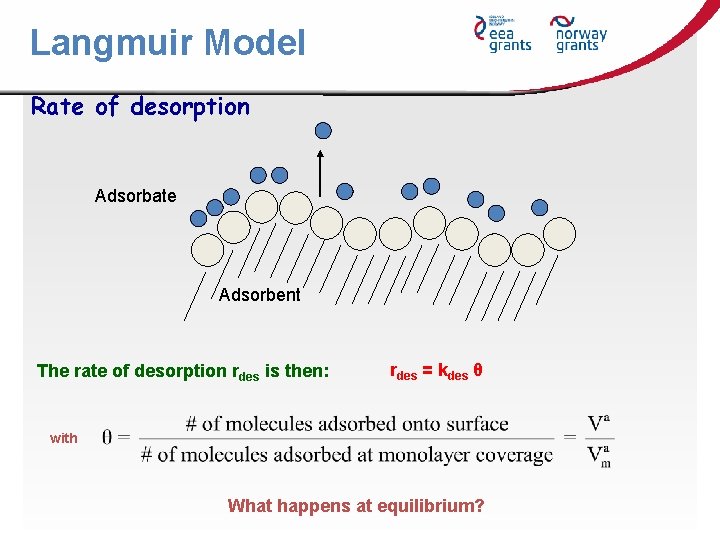
Langmuir Model Rate of desorption Adsorbate Adsorbent The rate of desorption rdes is then: rdes = kdes θ with What happens at equilibrium?

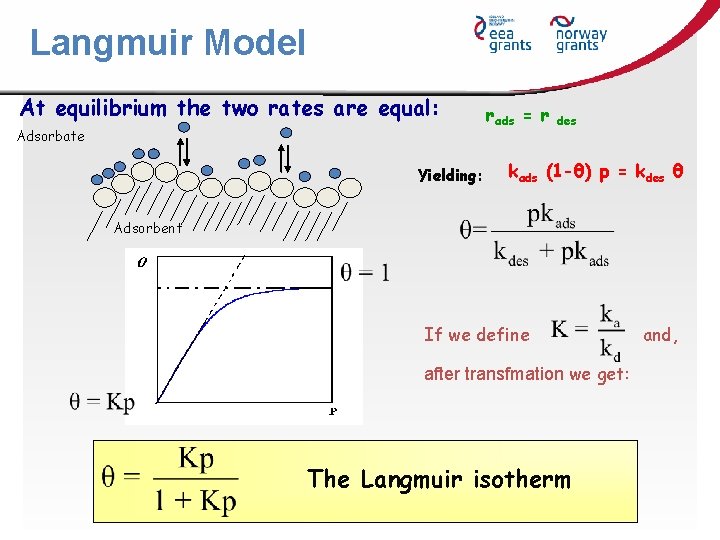
Langmuir Model At equilibrium the two rates are equal: Adsorbate Yielding: rads = r des kads (1 -θ) p = kdes θ Adsorbent If we define after transfmation we get: The Langmuir isotherm and,

Isotherm models: Stephen Brunauer BET Model: 1938 Paul H. Emmett Edward Teller Assumptions and limitations 1. All adsorption sites have the same energy (homogeneous surface). 2. No lateral interactions between adsorbate molecules. 3. The evaporation rate on a layer is equal to the condensation rate on the previous layer. 4. The surface is covered by 0, 1, 2, …, i, n layers; molecules occupy a fraction of surface θ 0, θ 1, θ 2, …, θi, θn.

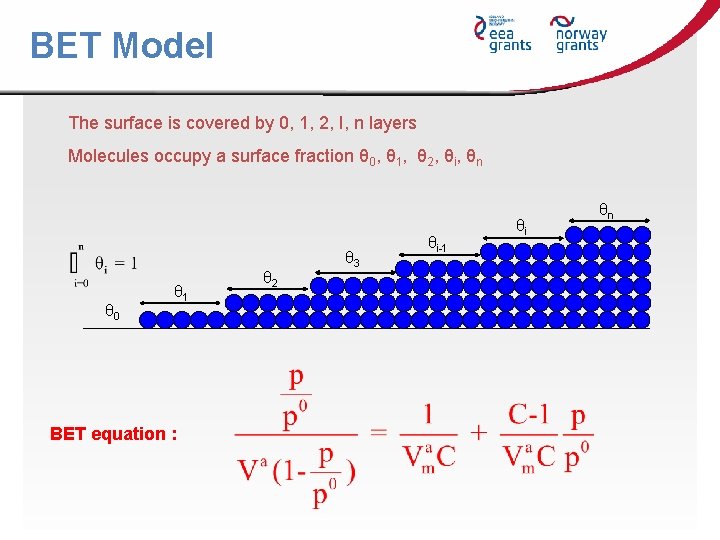
BET Model The surface is covered by 0, 1, 2, I, n layers Molecules occupy a surface fraction θ 0, θ 1, θ 2, θi, θn θ 3 θ 0 θ 1 BET equation : θ 2 θi-1 θi θn

Isotherm models: Determination of the morphology of a material : Characterization of its surface related to its extension → specific area related to its form → porosity, pores volume… The specific surface area is the accessible internal and external surface to an adsorptive for 1 gram of solid. m 2. g-1 The measure of the total pore volume gives the volume of the accessible pores from the adsorbed quantity for 1 gram of solid. cm 3. g-1

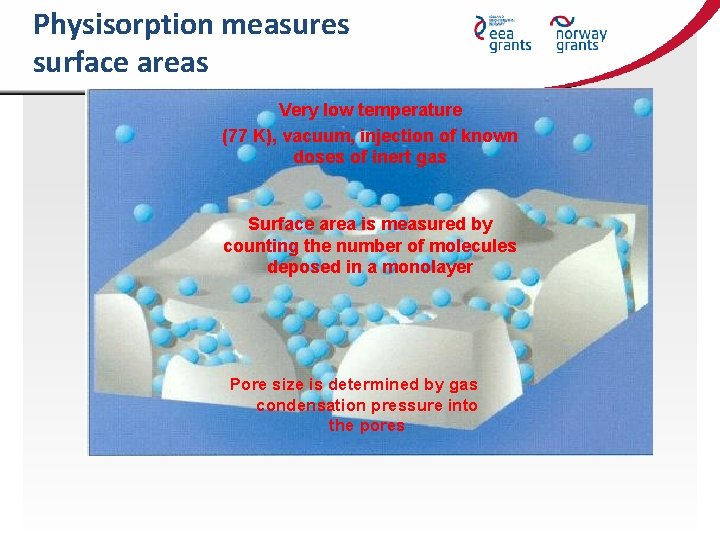
Physisorption measures surface areas Very low temperature (77 K), vacuum, injection of known doses of inert gas Surface area is measured by counting the number of molecules deposed in a monolayer Pore size is determined by gas condensation pressure into the pores

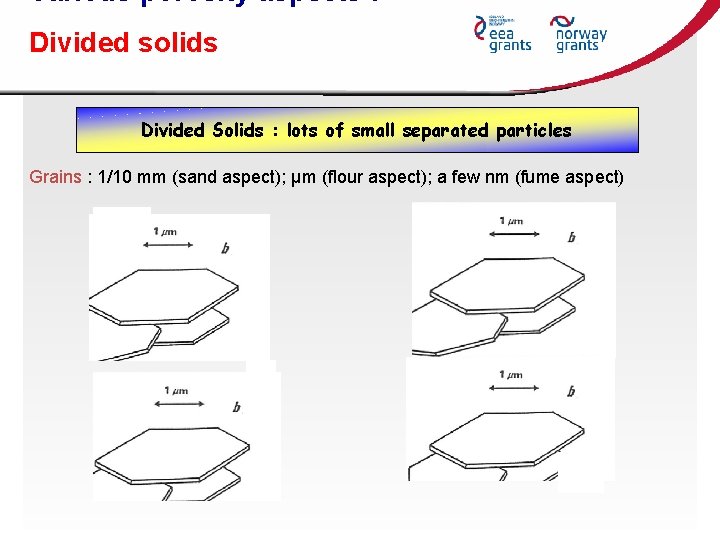
Various porosity aspects : Divided solids Divided Solids : lots of small separated particles Grains : 1/10 mm (sand aspect); μm (flour aspect); a few nm (fume aspect)

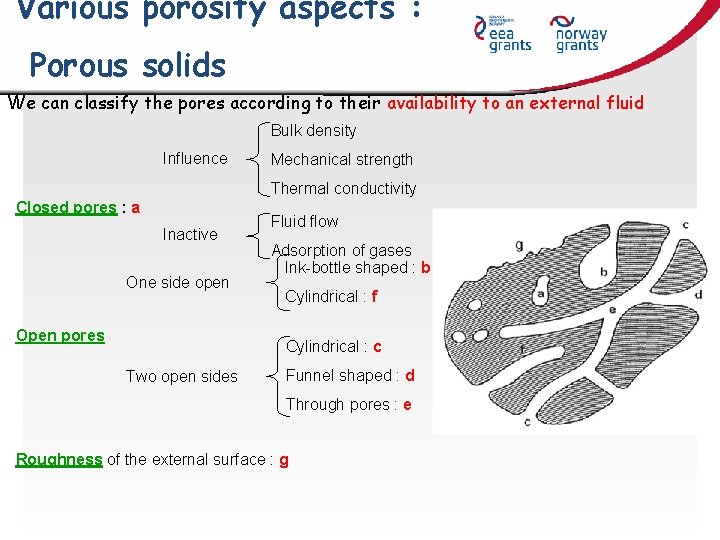
Various porosity aspects : Porous solids We can classify the pores according to their availability to an external fluid Bulk density Influence Mechanical strength Thermal conductivity Closed pores : a Inactive One side open Open pores Fluid flow Adsorption of gases Ink-bottle shaped : b Cylindrical : f Cylindrical : c Two open sides Funnel shaped : d Through pores : e Roughness of the external surface : g

Diverse porosity aspects : Porous solids P. Behrens, Adv. Mater. 1993, 5, 127

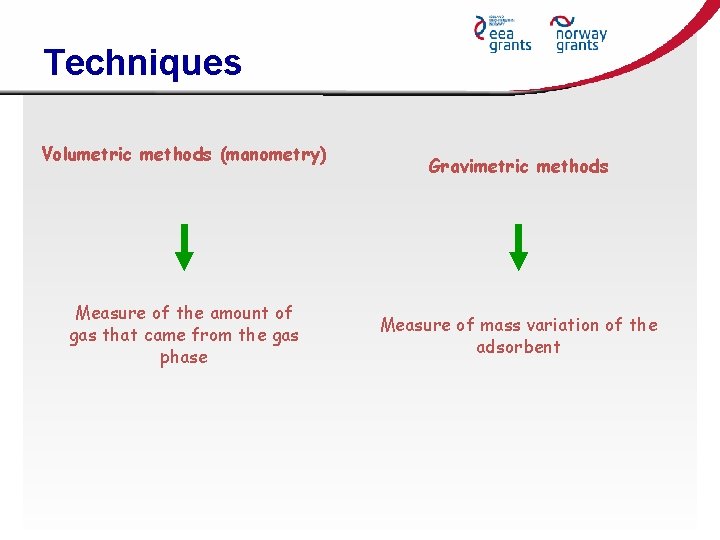
Techniques Volumetric methods (manometry) Measure of the amount of gas that came from the gas phase Gravimetric methods Measure of mass variation of the adsorbent

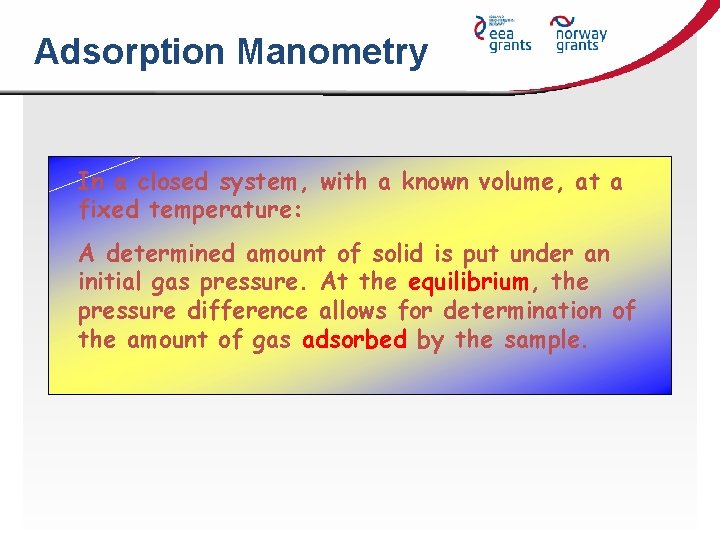
Adsorption Manometry In a closed system, with a known volume, at a fixed temperature: A determined amount of solid is put under an initial gas pressure. At the equilibrium, the pressure difference allows for determination of the amount of gas adsorbed by the sample.

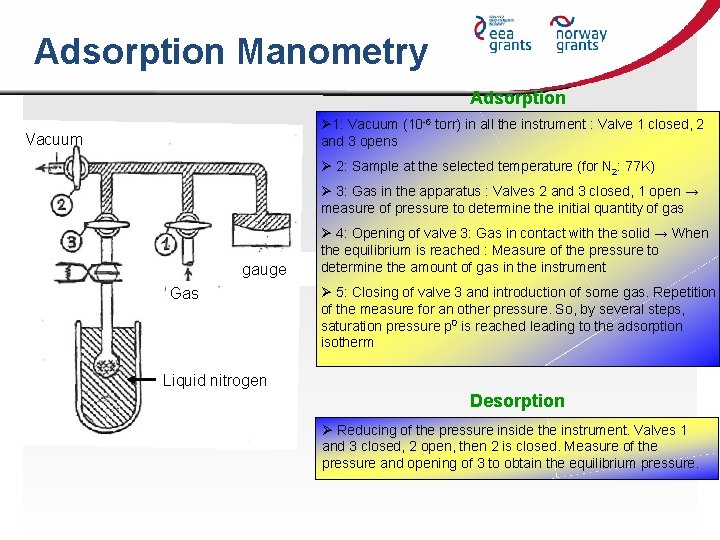
Adsorption Manometry Adsorption Ø 1: Vacuum (10 -6 torr) in all the instrument : Valve 1 closed, 2 and 3 opens Vacuum Ø 2: Sample at the selected temperature (for N 2: 77 K) Ø 3: Gas in the apparatus : Valves 2 and 3 closed, 1 open → measure of pressure to determine the initial quantity of gas gauge Gas Ø 4: Opening of valve 3: Gas in contact with the solid → When the equilibrium is reached : Measure of the pressure to determine the amount of gas in the instrument Ø 5: Closing of valve 3 and introduction of some gas. Repetition of the measure for an other pressure. So, by several steps, saturation pressure p 0 is reached leading to the adsorption isotherm Liquid nitrogen Desorption Ø Reducing of the pressure inside the instrument. Valves 1 and 3 closed, 2 open, then 2 is closed. Measure of the pressure and opening of 3 to obtain the equilibrium pressure.

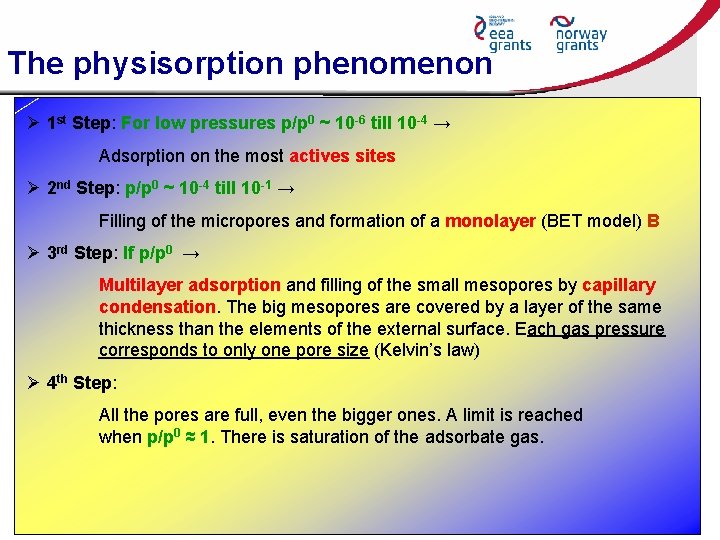
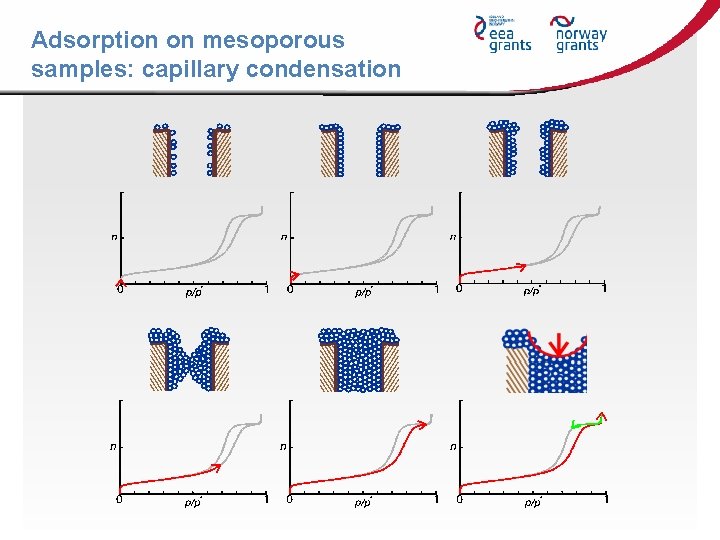
The physisorption phenomenon Ø 1 st Step: For low pressures p/p 0 ~ 10 -6 till 10 -4 → Adsorption on the most actives sites Ø 2 nd Step: p/p 0 ~ 10 -4 till 10 -1 → Filling of the micropores and formation of a monolayer (BET model) B Ø 3 rd Step: If p/p 0 → Multilayer adsorption and filling of the small mesopores by capillary condensation. The big mesopores are covered by a layer of the same thickness than the elements of the external surface. Each gas pressure corresponds to only one pore size (Kelvin’s law) Ø 4 th Step: All the pores are full, even the bigger ones. A limit is reached when p/p 0 ≈ 1. There is saturation of the adsorbate gas.

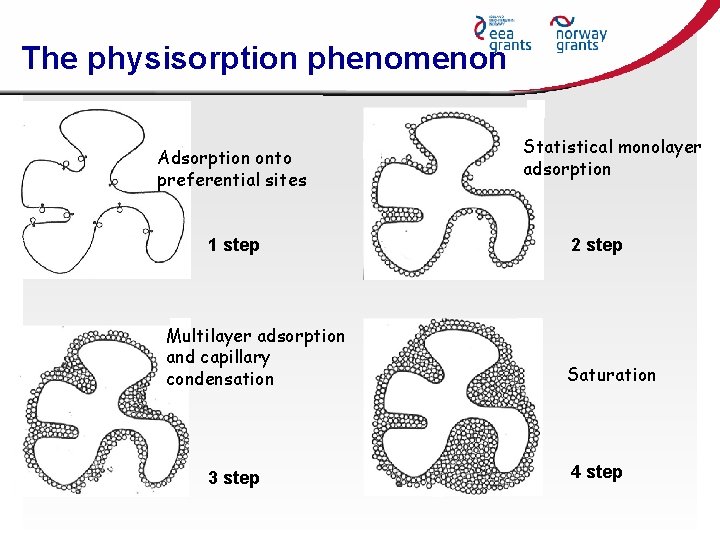
The physisorption phenomenon Adsorption onto preferential sites 1 step Multilayer adsorption and capillary condensation 3 step Statistical monolayer adsorption 2 step Saturation 4 step

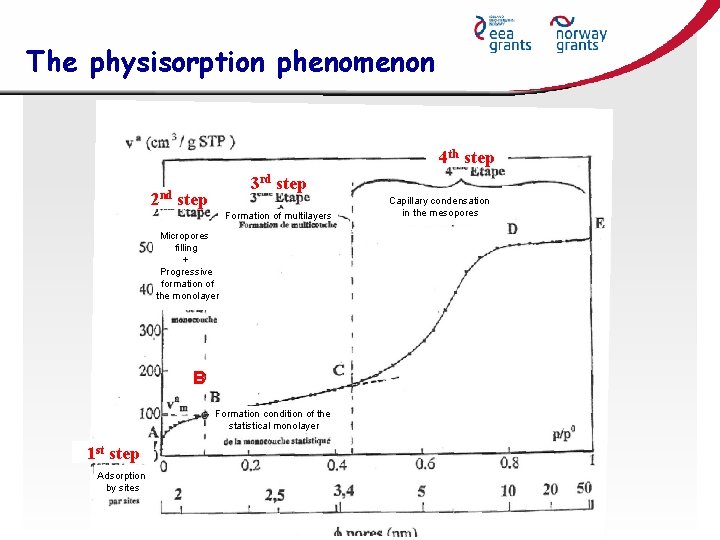
The physisorption phenomenon 4 th step 3 rd step 2 nd step Formation of multilayers Micropores filling + Progressive formation of the monolayer B Formation condition of the statistical monolayer 1 st step Adsorption by sites Capillary condensation in the mesopores

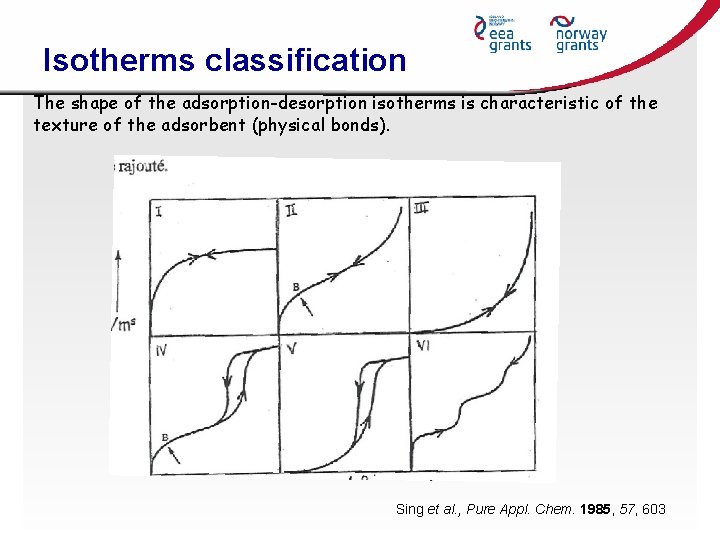
Isotherms classification The shape of the adsorption-desorption isotherms is characteristic of the texture of the adsorbent (physical bonds). Sing et al. , Pure Appl. Chem. 1985, 57, 603

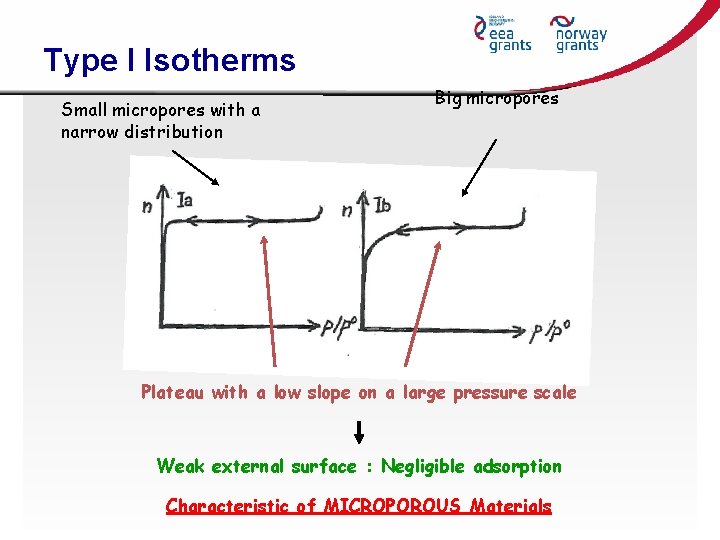
Type I Isotherms Small micropores with a narrow distribution Big micropores Plateau with a low slope on a large pressure scale Weak external surface : Negligible adsorption Characteristic of MICROPOROUS Materials

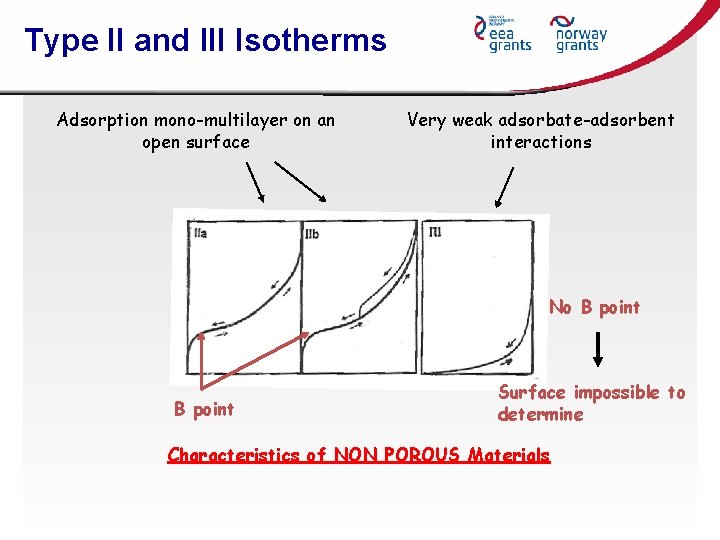
Type II and III Isotherms Adsorption mono-multilayer on an open surface Very weak adsorbate-adsorbent interactions No B point Surface impossible to determine Characteristics of NON POROUS Materials

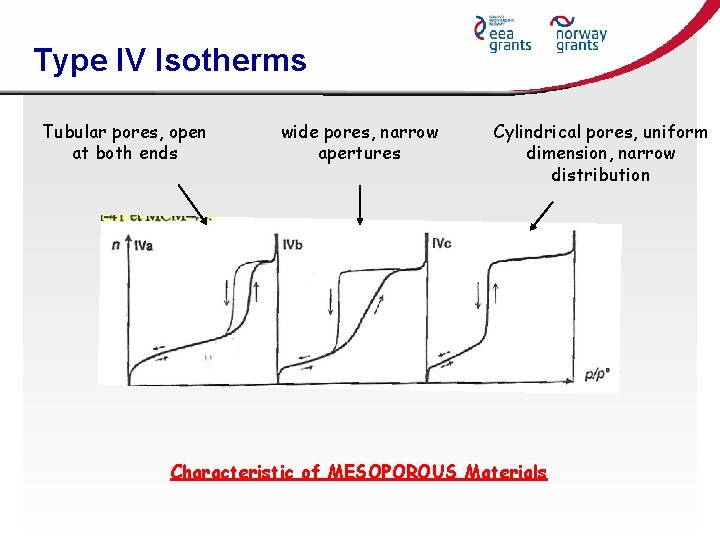
Type IV Isotherms Tubular pores, open at both ends wide pores, narrow apertures Cylindrical pores, uniform dimension, narrow distribution Characteristic of MESOPOROUS Materials

Adsorption on mesoporous samples: capillary condensation

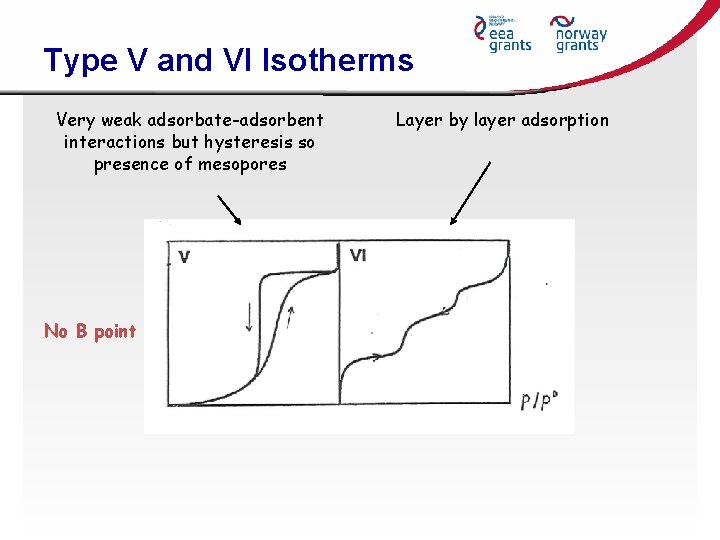
Type V and VI Isotherms Very weak adsorbate-adsorbent interactions but hysteresis so presence of mesopores No B point Layer by layer adsorption

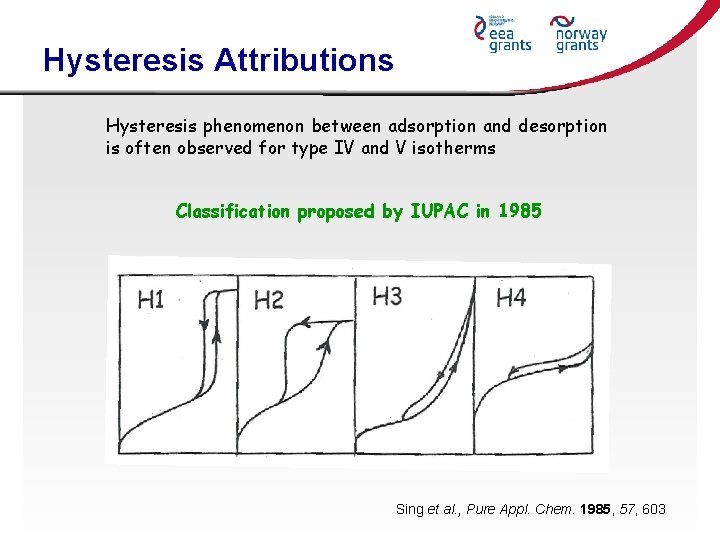
Hysteresis Attributions Hysteresis phenomenon between adsorption and desorption is often observed for type IV and V isotherms Classification proposed by IUPAC in 1985 Sing et al. , Pure Appl. Chem. 1985, 57, 603

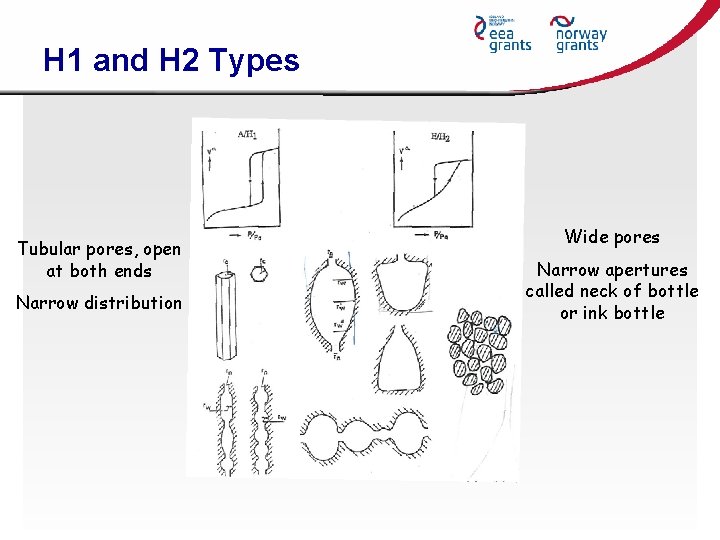
H 1 and H 2 Types Tubular pores, open at both ends Narrow distribution Wide pores Narrow apertures called neck of bottle or ink bottle

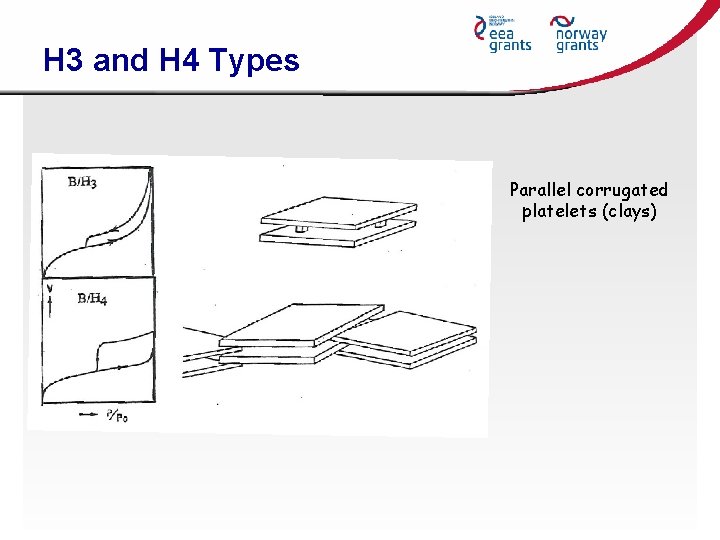
H 3 and H 4 Types Parallel corrugated platelets (clays)

Thank you!! This project is funded by the Norwegian Financial Mechanism. Registration number: NF-CZ 07 ICP-1 -040 -2014. Name of the project: „Formation of research surrounding for young researchers in the field of advanced materials for catalysis and bioapplications“