CHARACTERISTIC PROPERTIES OF MATTER PHYSICAL CHARACTERISTICS MELTING POINT

- Slides: 9

CHARACTERISTIC PROPERTIES OF MATTER PHYSICAL CHARACTERISTICS MELTING POINT - BOILING POINT - DENSITY - SOLUBILITY - CHEMICAL CHARACTERISTICS • REACTIONS TO INDICATORS

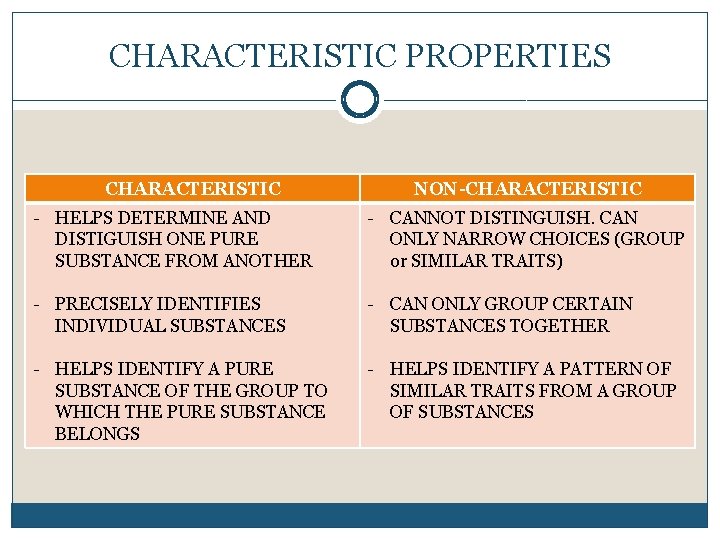
CHARACTERISTIC PROPERTIES CHARACTERISTIC NON-CHARACTERISTIC - HELPS DETERMINE AND DISTIGUISH ONE PURE SUBSTANCE FROM ANOTHER - CANNOT DISTINGUISH. CAN ONLY NARROW CHOICES (GROUP or SIMILAR TRAITS) - PRECISELY IDENTIFIES INDIVIDUAL SUBSTANCES - CAN ONLY GROUP CERTAIN SUBSTANCES TOGETHER - HELPS IDENTIFY A PURE SUBSTANCE OF THE GROUP TO WHICH THE PURE SUBSTANCE BELONGS - HELPS IDENTIFY A PATTERN OF SIMILAR TRAITS FROM A GROUP OF SUBSTANCES

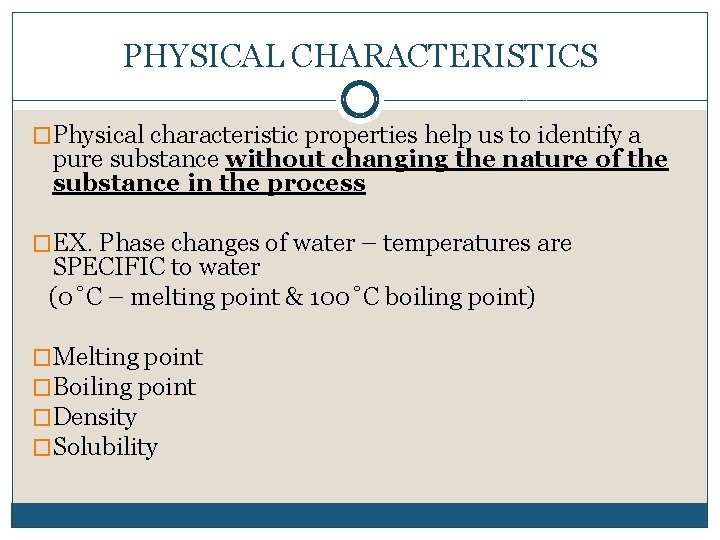
PHYSICAL CHARACTERISTICS �Physical characteristic properties help us to identify a pure substance without changing the nature of the substance in the process �EX. Phase changes of water – temperatures are SPECIFIC to water (0˚C – melting point & 100˚C boiling point) �Melting point �Boiling point �Density �Solubility

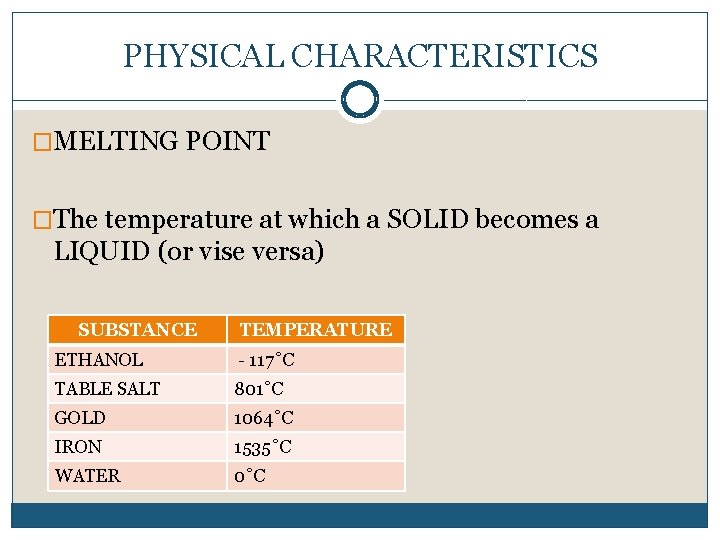
PHYSICAL CHARACTERISTICS �MELTING POINT �The temperature at which a SOLID becomes a LIQUID (or vise versa) SUBSTANCE TEMPERATURE ETHANOL - 117˚C TABLE SALT 801˚C GOLD 1064˚C IRON 1535˚C WATER 0˚C

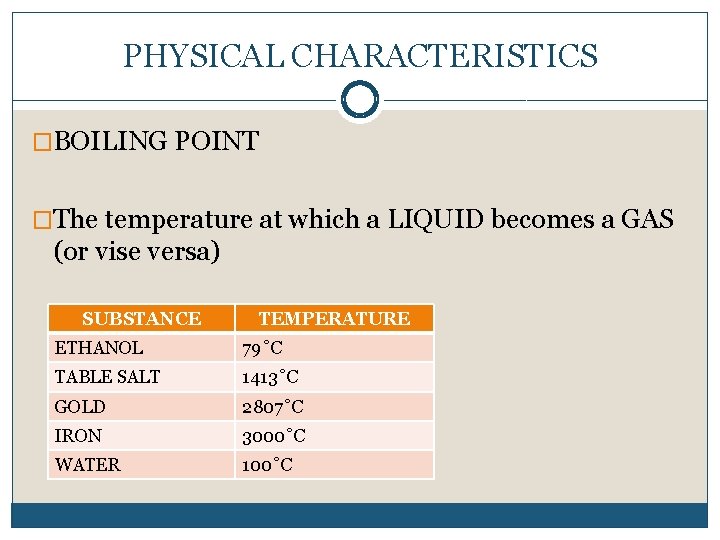
PHYSICAL CHARACTERISTICS �BOILING POINT �The temperature at which a LIQUID becomes a GAS (or vise versa) SUBSTANCE TEMPERATURE ETHANOL 79˚C TABLE SALT 1413˚C GOLD 2807˚C IRON 3000˚C WATER 100˚C

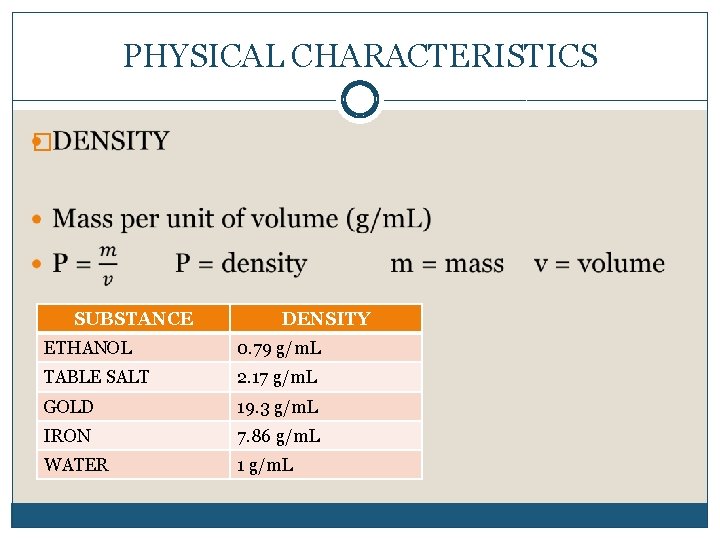
PHYSICAL CHARACTERISTICS � SUBSTANCE DENSITY ETHANOL 0. 79 g/m. L TABLE SALT 2. 17 g/m. L GOLD 19. 3 g/m. L IRON 7. 86 g/m. L WATER 1 g/m. L

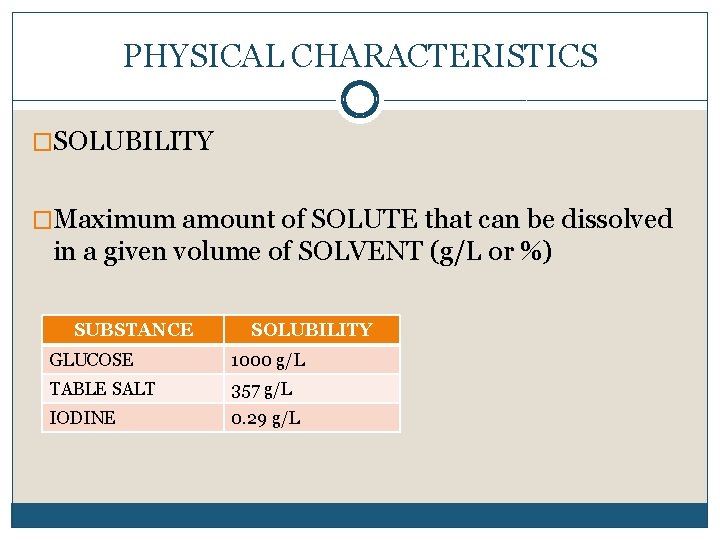
PHYSICAL CHARACTERISTICS �SOLUBILITY �Maximum amount of SOLUTE that can be dissolved in a given volume of SOLVENT (g/L or %) SUBSTANCE SOLUBILITY GLUCOSE 1000 g/L TABLE SALT 357 g/L IODINE 0. 29 g/L

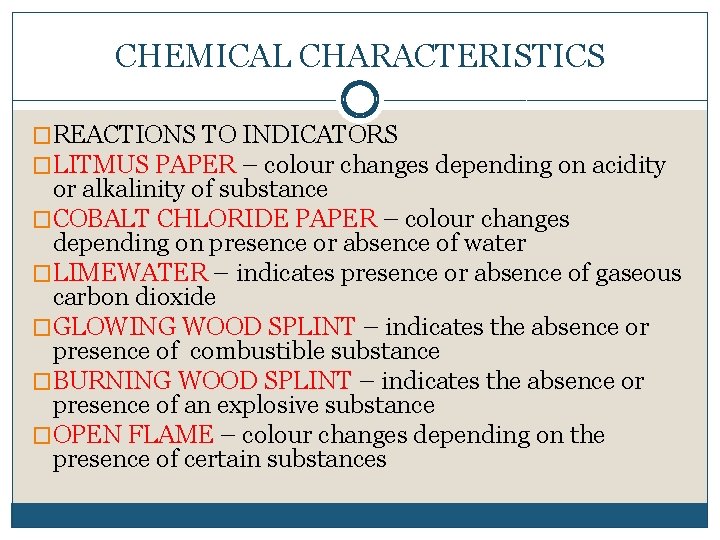
CHEMICAL CHARACTERISTICS �REACTIONS TO INDICATORS �LITMUS PAPER – colour changes depending on acidity or alkalinity of substance �COBALT CHLORIDE PAPER – colour changes depending on presence or absence of water �LIMEWATER – indicates presence or absence of gaseous carbon dioxide �GLOWING WOOD SPLINT – indicates the absence or presence of combustible substance �BURNING WOOD SPLINT – indicates the absence or presence of an explosive substance �OPEN FLAME – colour changes depending on the presence of certain substances

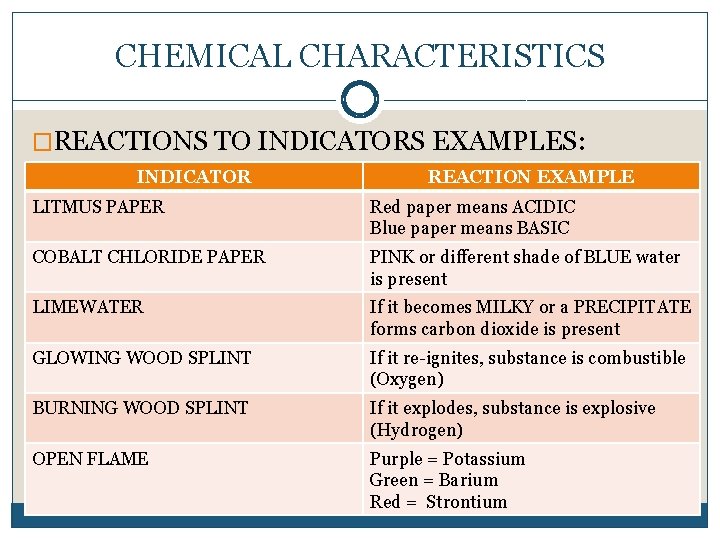
CHEMICAL CHARACTERISTICS �REACTIONS TO INDICATORS EXAMPLES: INDICATOR REACTION EXAMPLE LITMUS PAPER Red paper means ACIDIC Blue paper means BASIC COBALT CHLORIDE PAPER PINK or different shade of BLUE water is present LIMEWATER If it becomes MILKY or a PRECIPITATE forms carbon dioxide is present GLOWING WOOD SPLINT If it re-ignites, substance is combustible (Oxygen) BURNING WOOD SPLINT If it explodes, substance is explosive (Hydrogen) OPEN FLAME Purple = Potassium Green = Barium Red = Strontium