Characteristic and Formation of Hydroxyapatite Synthesized from Heat



- Slides: 21

Characteristic and Formation of Hydroxyapatite Synthesized from Heat Treatment of Cuttlefish Bone Kridsada Faksa Department of Physics, Faculty o King Mongkut’s University of Technolo

Outlines Introduction bone Results & Discussion 2 Objective s Experime nt Conclusio n

Introduction 3 Ref: http: //eng. thesaurus. rusnano. com 0 Dimensi on 1 Dimensi on 2 Dimens ion 3 Dimensi on Ref: http: //commonsensecanadian. co m Nano scale 1 to 100 nanometers Shape and Size Effect Increasing of specific surface area 3

Introduction 4 ü Enhanced resorbability Bone ü Improved densification ü sinter ability Hydroxyapa tite ü Improved cell proliferation ü Improved cellular activity related to bone growth Ref: http: //oncoinfo. ru Ref: http: //i. vimeocdn. c

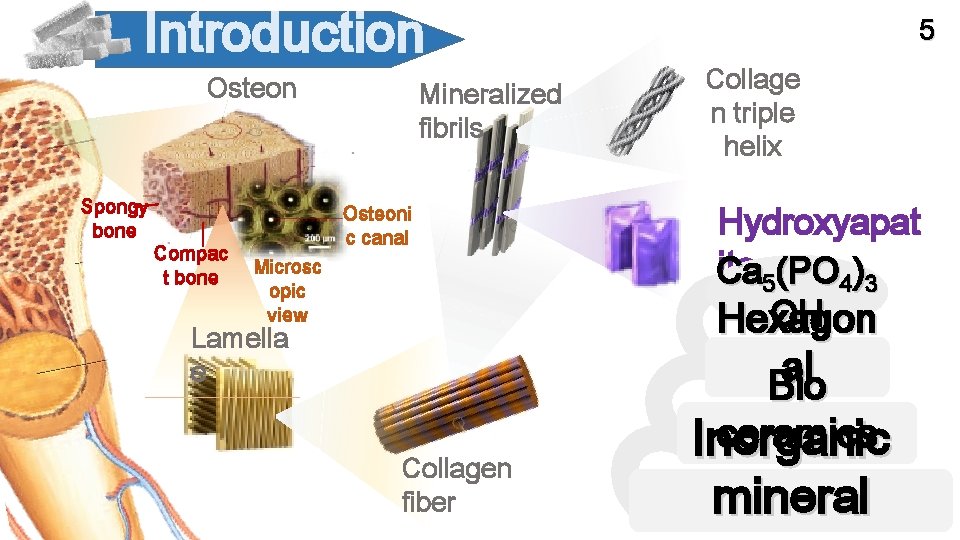
Introduction Osteon Spongy bone Compac t bone Mineralized fibrils Osteoni c canal Microsc opic view Lamella e Collagen fiber 5 Collage n triple helix Hydroxyapat ite 5(PO 4)3 Ca OH Hexagon al Bio ceramics Inorganic mineral

Introduction How do you synthesized hydroxyapatite? q Natural material Ca source PO 4 source 90 -95% of Ca q Chemical material K 2 HPO Ca 2 P 2 O 4 7 Trace Na 2 HP O 4 )NH 2(4 H PO 4 Found β - Trace of Na Odor 6

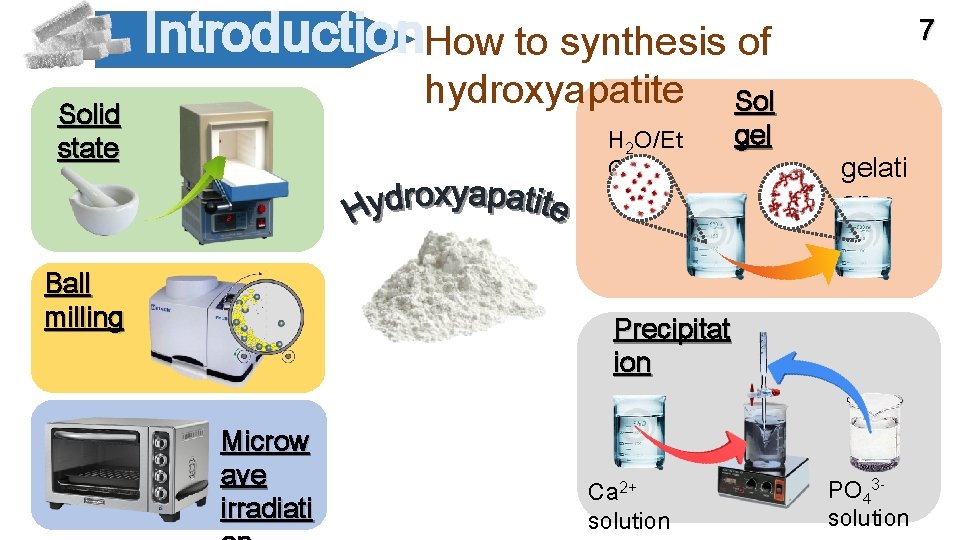
Introduction. How to synthesis of hydroxyapatite Solid state H 2 O/Et OH Ball milling Sol gel 7 gelati on Precipitat ion Microw ave irradiati Ca 2+ solution PO 437 solution

Objectives q To study phase transformation of cuttlefish bone by various sintering temperature. q To study characteristics of hydroxyapatite synthesized from various heated cuttlefish bone by ball milling method. q To study crystal structure, functional group and morphology of synthesized hydroxyapatite by ball milling method. 8

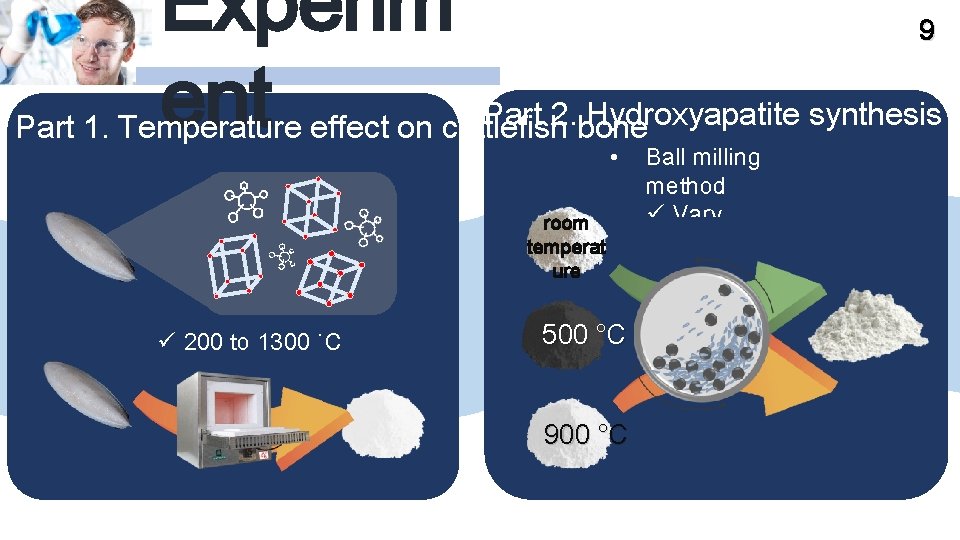
Experim ent 9 Part 2. bone Hydroxyapatite synthesis Part 1. Temperature effect on cuttlefish • room temperat ure ü 200 to 1300 ˚C 500 °C 900 °C Ball milling method ü Vary precursor

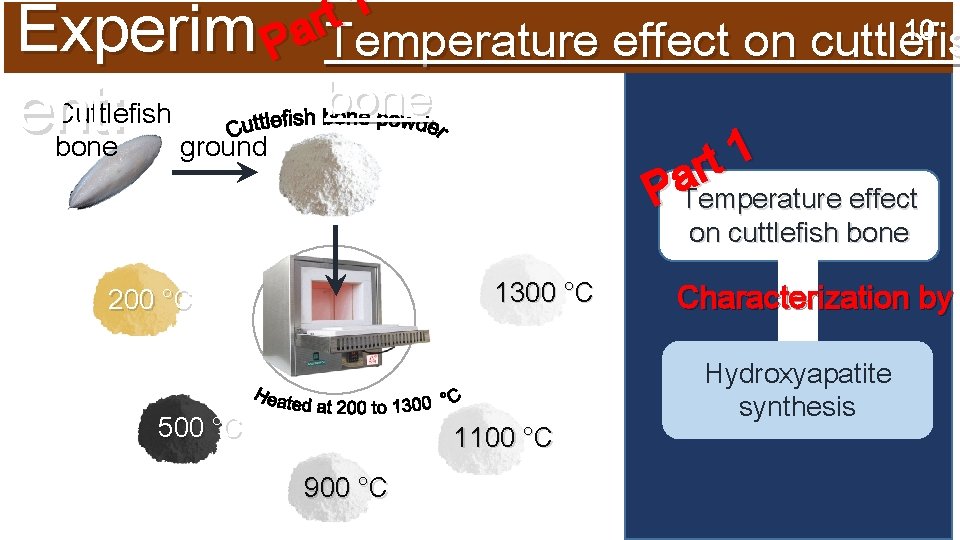
1 t 10 Experim Par. Temperature effect on cuttlefis ent: Cuttlefish ground bone 1 t r a P Temperature effect on cuttlefish bone 1300 °C 200 °C 500 °C 1100 °C 900 °C Characterization by Hydroxyapatite synthesis

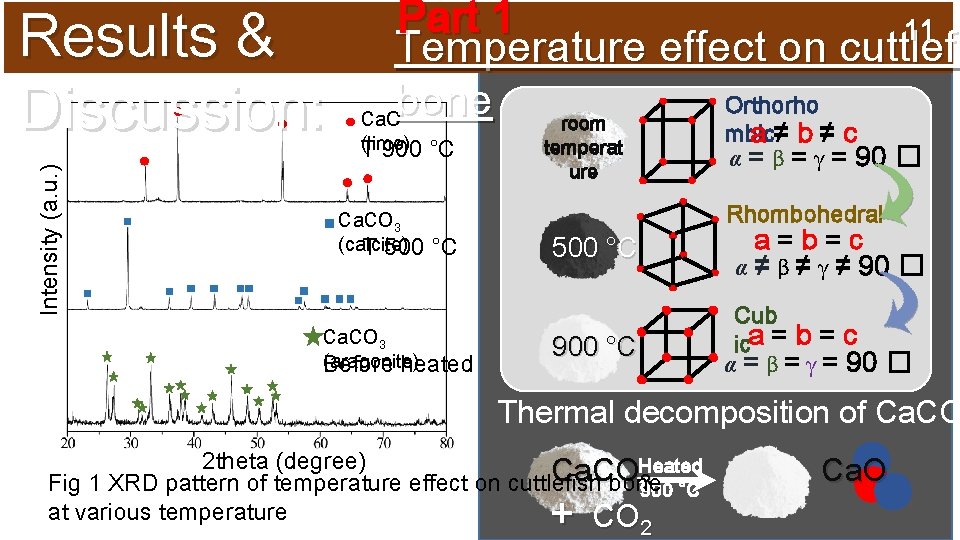
Intensity (a. u. ) Results & Discussion: Part 1 11 Temperature effect on cuttlefi Orthorho bone Ca. O room (lime) T 900 Ca. CO 3 (calcite) T 500 °C mbic a≠b≠c α = β = γ = 90 � temperat ure Rhombohedral °C Ca. CO 3 (aragonite) Before heated a=b=c α ≠ β ≠ γ ≠ 90 � 500 °C Cub ica = b = c α = β = γ = 90 � 900 °C Thermal decomposition of Ca. CO 2 theta (degree) Heated Ca. CO 3 Fig 1 XRD pattern of temperature effect on cuttlefish bone 900 °C at various temperature CO + 2 Ca. O

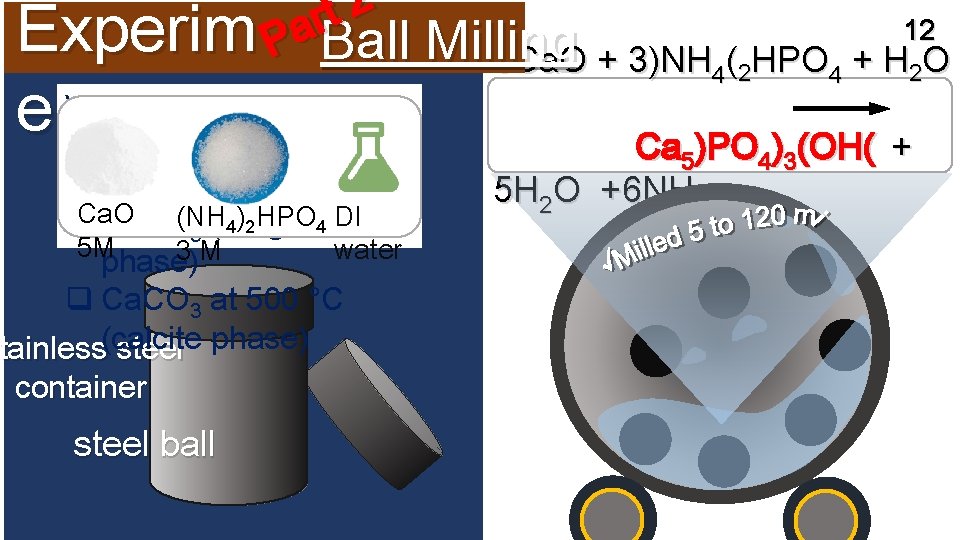
2 t r 12 a Experim P Ball Milling 5 Ca. O + 3)NH 4(2 HPO 4 + H 2 O Vary calcium sources ent: q Ca. O (lime phase) (for example) Ca. O )2 HPO 4 DI 4 q Ca. CO(NH (aragonite 3 5 M water 3 M phase) q Ca. CO 3 at 500 °C tainless(calcite steel phase) Stainless container steel ball Ca 5)PO 4)3(OH( + 5 H 2 O +6 NH 3

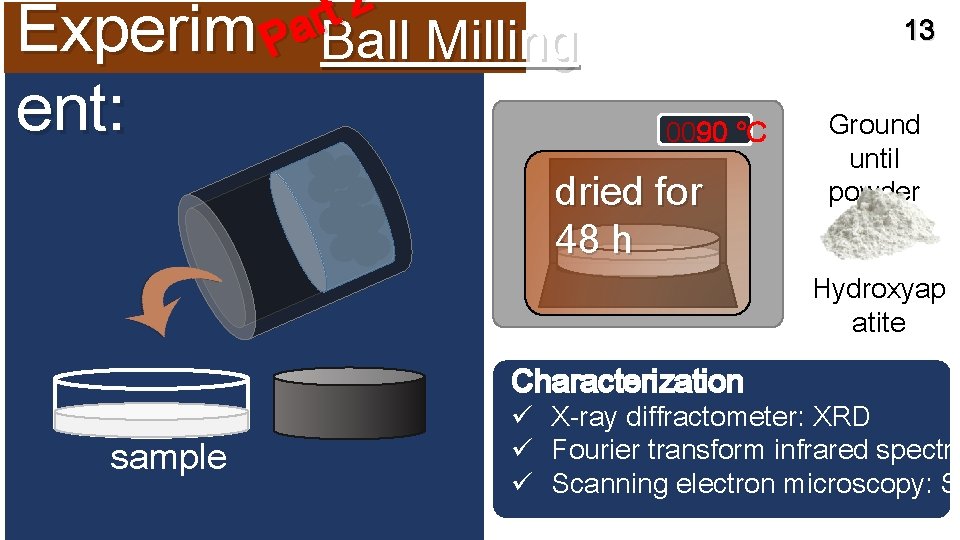
2 t r a Experim P Ball Milling ent: 13 0090 °C dried for 48 h Ground until powder Hydroxyap atite Characterization sample ü X-ray diffractometer: XRD ü Fourier transform infrared spectro ü Scanning electron microscopy: SE

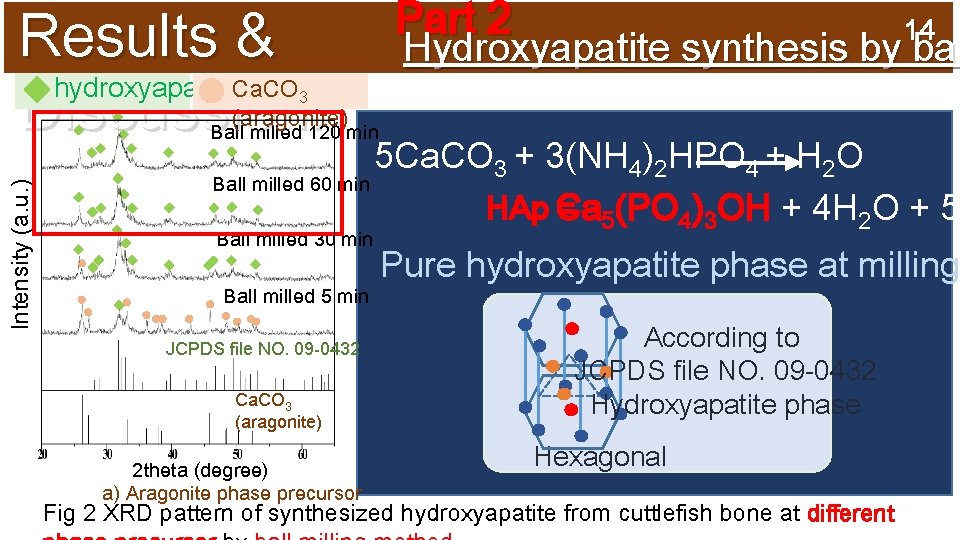
Results & hydroxyapatite Discussion: Part 2 14 Hydroxyapatite synthesis by bal Ca. CO 3 (aragonite) Intensity (a. u. ) Ball milled 120 min 5 Ca. CO 3 + 3(NH 4)2 HPO 4 + H 2 O Ball milled 60 min HAp Ca = 5(PO 4)3 OH + 4 H 2 O + 5 Ball milled 30 min Ball milled 5 min JCPDS file NO. 09 -0432 Ca. CO 3 (aragonite) 2 theta (degree) a) Aragonite phase precursor Pure hydroxyapatite phase at milling According to JCPDS file NO. 09 -0432 Hydroxyapatite phase Hexagonal Fig 2 XRD pattern of synthesized hydroxyapatite from cuttlefish bone at different

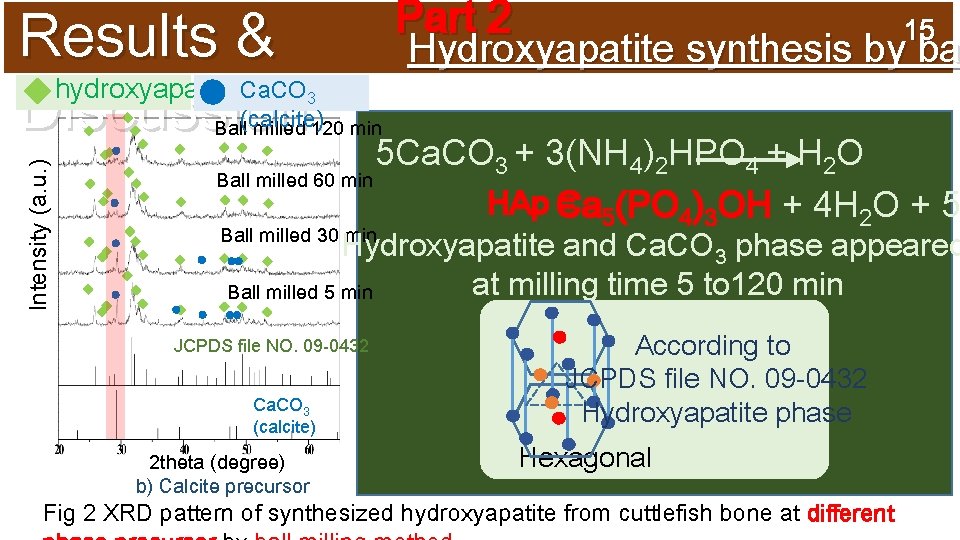
Results & hydroxyapatite Discussion: Part 2 15 Hydroxyapatite synthesis by ba Intensity (a. u. ) Ca. CO 3 Ball(calcite) milled 120 min 5 Ca. CO 3 + 3(NH 4)2 HPO 4 + H 2 O Ball milled 60 min HAp Ca = 5(PO 4)3 OH + 4 H 2 O + 5 Ball milled 30 min Hydroxyapatite and Ca. CO 3 phase appeared at milling time 5 to 120 min Ball milled 5 min JCPDS file NO. 09 -0432 Ca. CO 3 (calcite) 2 theta (degree) b) Calcite precursor According to JCPDS file NO. 09 -0432 Hydroxyapatite phase Hexagonal Fig 2 XRD pattern of synthesized hydroxyapatite from cuttlefish bone at different

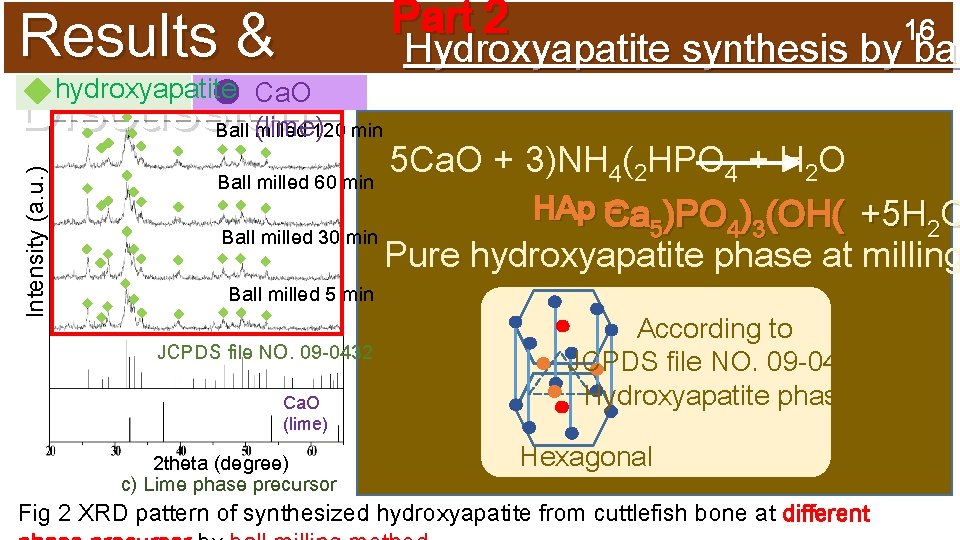
Results & hydroxyapatite Ca. O Discussion: (lime) Intensity (a. u. ) Ball milled 120 min Ball milled 60 min Ball milled 30 min Part 2 16 Hydroxyapatite synthesis by bal 5 Ca. O + 3)NH 4(2 HPO 4 + H 2 O HAp Ca = )PO ) (OH( +5 H O 5 4 3 2 Pure hydroxyapatite phase at milling Ball milled 5 min JCPDS file NO. 09 -0432 Ca. O (lime) 2 theta (degree) c) Lime phase precursor According to JCPDS file NO. 09 -0432 Hydroxyapatite phase Hexagonal Fig 2 XRD pattern of synthesized hydroxyapatite from cuttlefish bone at different

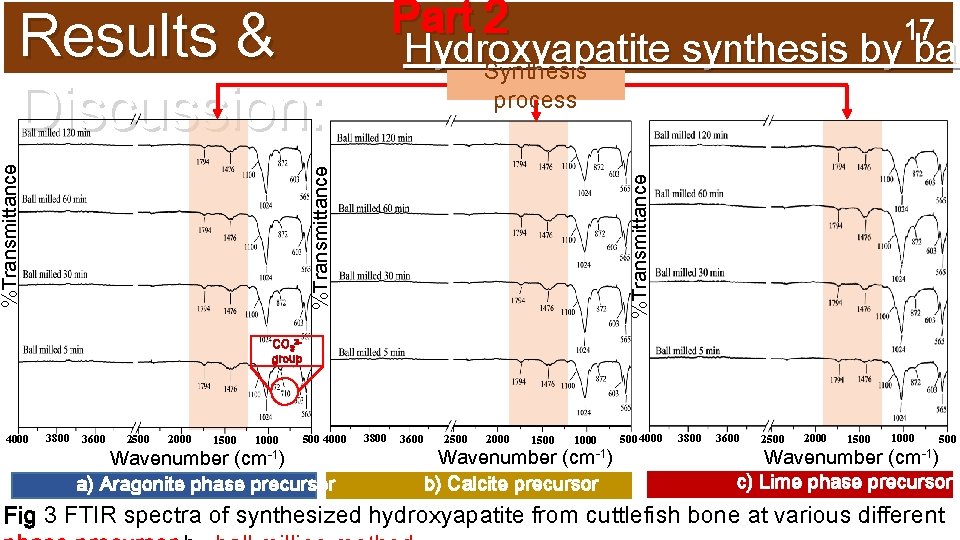
17 Hydroxyapatite synthesis by bal Synthesis process %Transmittance Results & Discussion: Part 2 CO 32 group 4000 3800 3600 2500 2000 1500 1000 500 4000 3800 3600 Wavenumber (cm-1) a) Aragonite phase precursor 2500 2000 1500 1000 Wavenumber (cm-1) b) Calcite precursor 5004000 3800 3600 2500 2000 1500 1000 500 Wavenumber (cm-1) c) Lime phase precursor Fig 3 FTIR spectra of synthesized hydroxyapatite from cuttlefish bone at various different

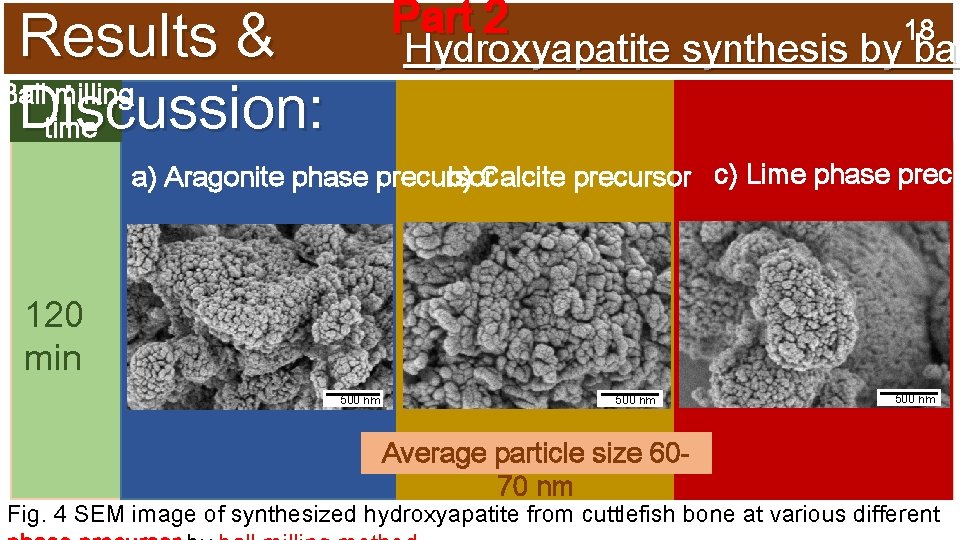
Part 2 Results & Ball milling Discussion: time 18 Hydroxyapatite synthesis by bal a) Aragonite phase precursor b) Calcite precursor c) Lime phase precu 120 min 500 nm Average particle size 6070 nm 500 nm Fig. 4 SEM image of synthesized hydroxyapatite from cuttlefish bone at various different

Conclusions Conclusions From results of temperature effect on cuttlefish Conclusions Conclusi Conclus Aragonite phase change to calcite phase completely and calcite phase transform to lime phase completely at temperature 500 °C and. Conclusions 900 °C respectively. From results of hydroxyapatite synthesis Hydroxyapatite phase appear at milling 5 minutes Conclusions Conclu 19 • Hydroxyapatite phase appear completely at 60 minutes in Ca. CO 3 (aragonite phase) precursor. • Hydroxyapatite phase appear completely at more than 120 minutes in Ca. CO 3 (calcite phase) precursor • Hydroxyapatite phase appear completely at 5 minutes in Ca. O (lime) precursor Conclusions

References ü Amin, S. , Bekhit, A. E. , Azam, A. and Zhifa, S. , 2015, “Synthesis of Nano- Hydroxyapatite (n. HA) from Waste Mussel Shells Using a Rapid Microwave Method”, Materials Chemistry and Physics, Vol. 149 -150, pp. 607ü Mehdi, S. S. , Khorasani, M. T. , Ehsan, D. K. and Ahmad, J. , 616. 2013, “Synthesis Methods for Nanosized Hydroxyapatite 20 Indiverse Structures ”, Acta Biomaterialia, Vol. 4, pp. 281ü 312. Liu, J. , Li , K. , Wang, H. , Zhu, M. and Yan, H. , 2014, “Rapid Formation of Hydroxyapatite Nanostructures by Microwave Irradiation”, Chemical Physical, Vol. 396, pp.
