Chapters 9 Molecular Geometry Bonding Theories Lewis structures
Chapters 9 Molecular Geometry & Bonding Theories
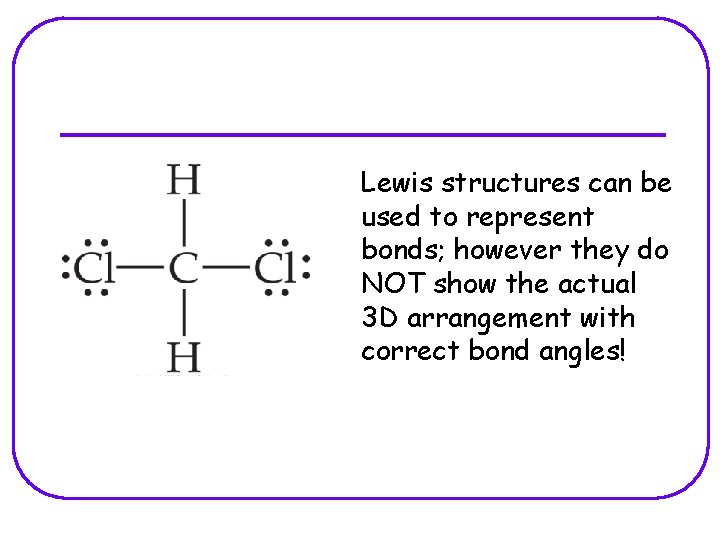
Lewis structures can be used to represent bonds; however they do NOT show the actual 3 D arrangement with correct bond angles!
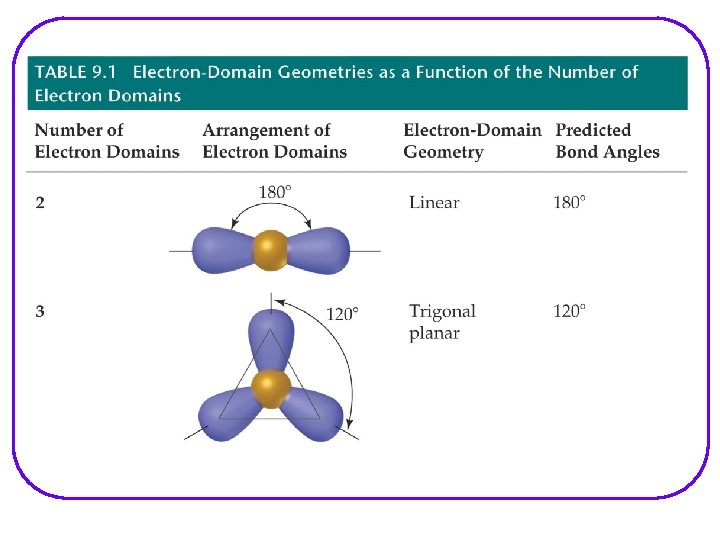
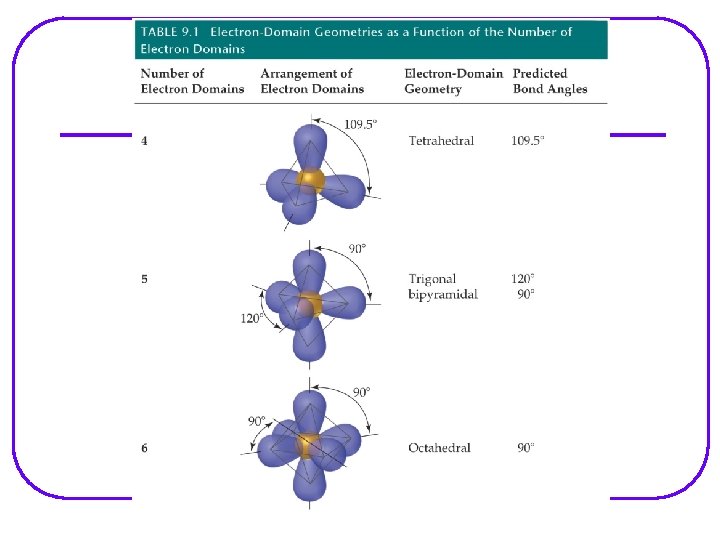
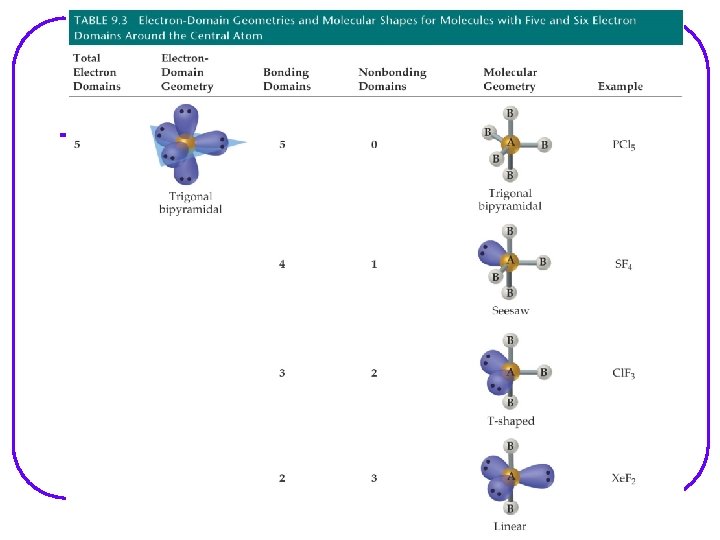
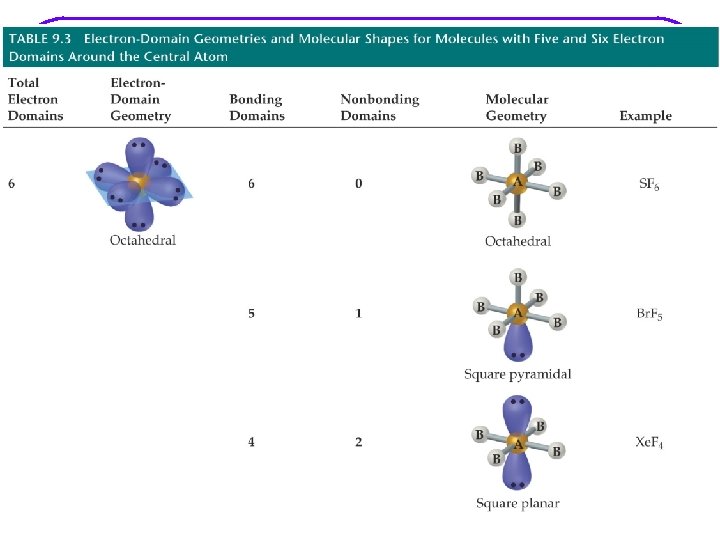
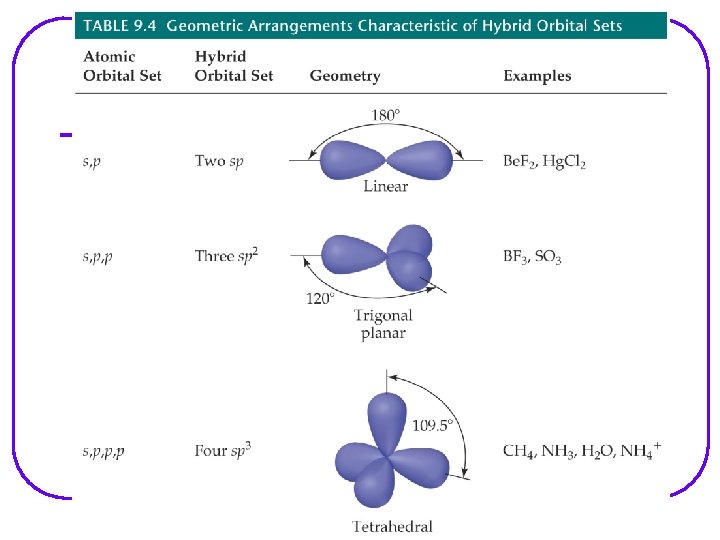
VSEPR Valence Shell Electron – pair Repulsion Model l Provides a description of the valence e- arrangement in the molecule bases on Lewis structures. Predicts the geometry of the molecule bases on e - pair repulsion. Including: • e- geometry : e- pairs repel each other and will assume orientation about the atom to minimize repulsion. • Molecular geometry • Bond angles Provides a description of the type of atomic orbitals used by atoms to share e- or to hold lone pair e- (nonbonding e-)
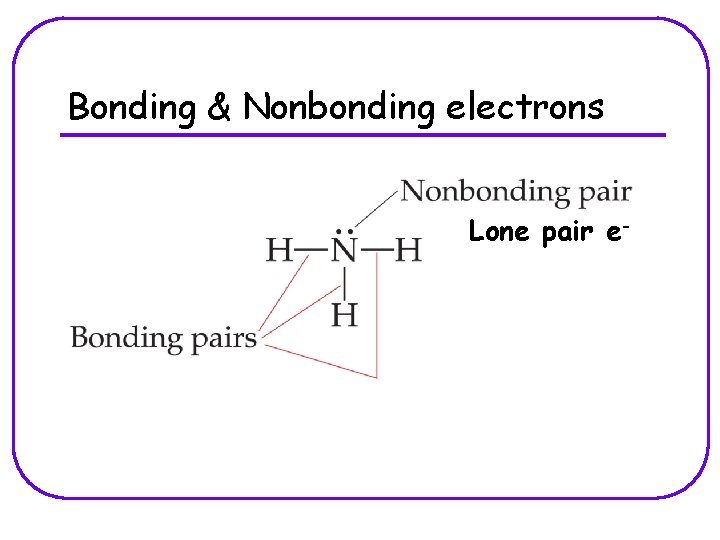
Bonding & Nonbonding electrons Lone pair e-
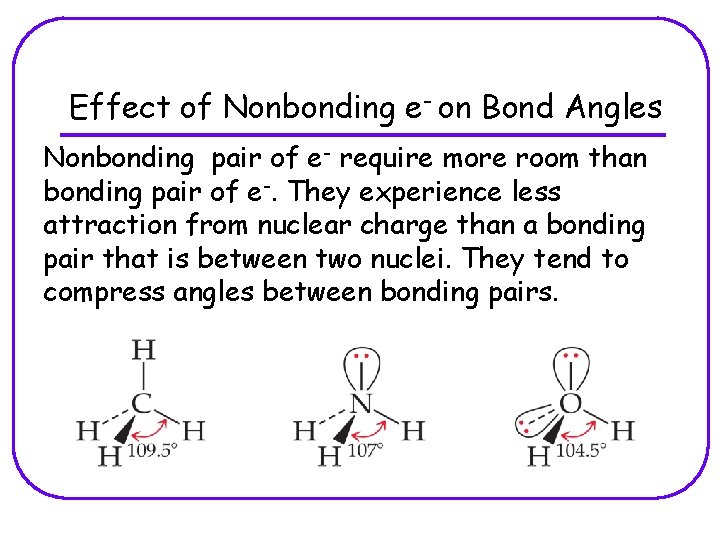
Effect of Nonbonding e- on Bond Angles Nonbonding pair of e- require more room than bonding pair of e-. They experience less attraction from nuclear charge than a bonding pair that is between two nuclei. They tend to compress angles between bonding pairs.
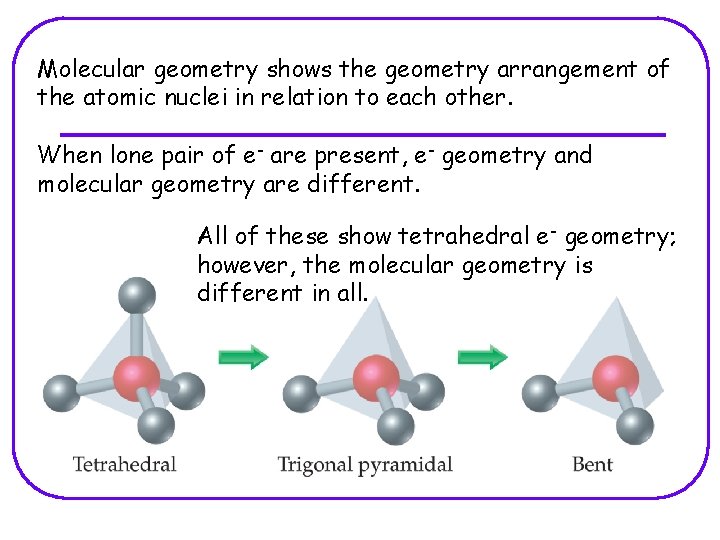
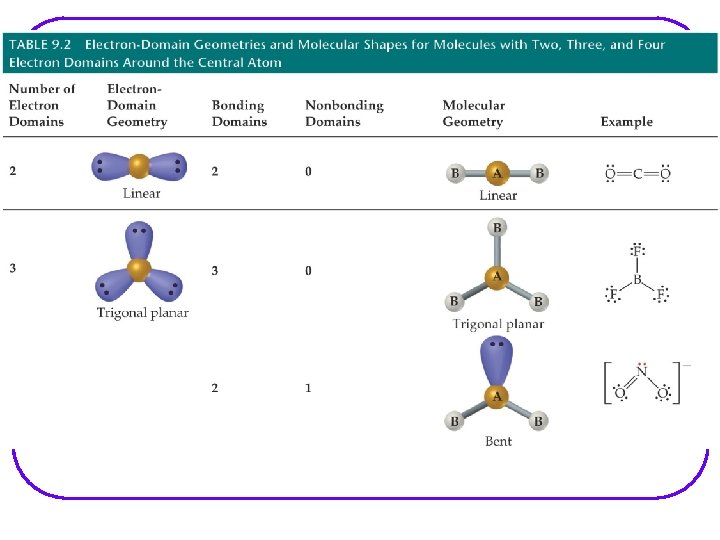
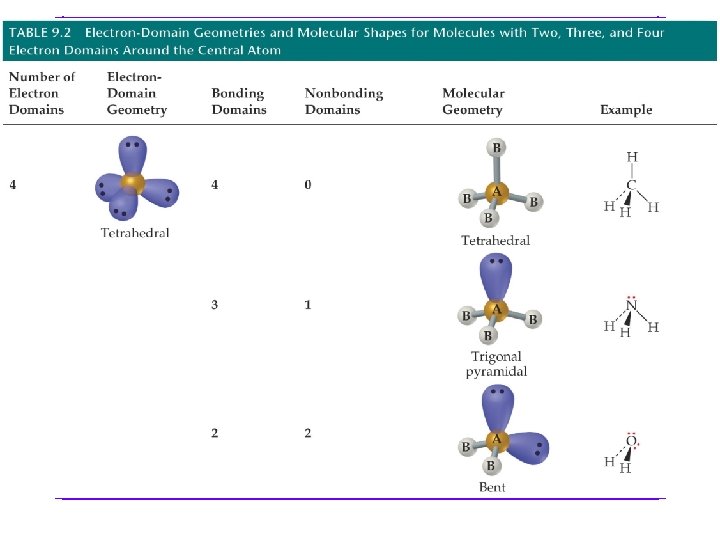
Molecular geometry shows the geometry arrangement of the atomic nuclei in relation to each other. When lone pair of e- are present, e- geometry and molecular geometry are different. All of these show tetrahedral e- geometry; however, the molecular geometry is different in all.
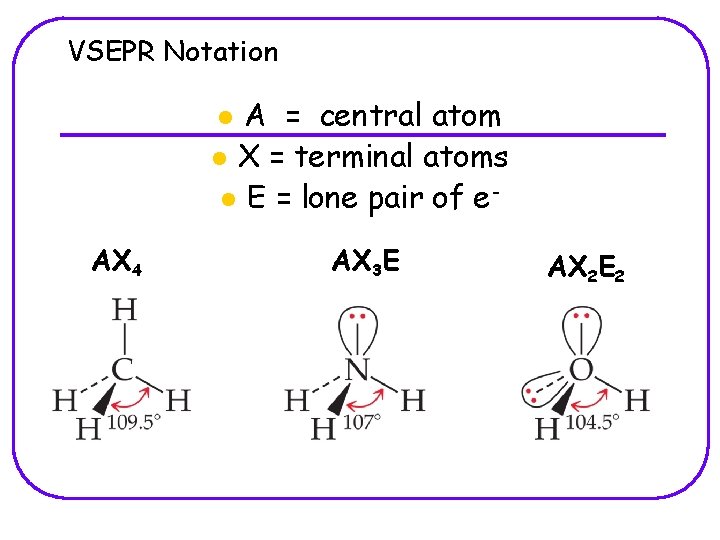
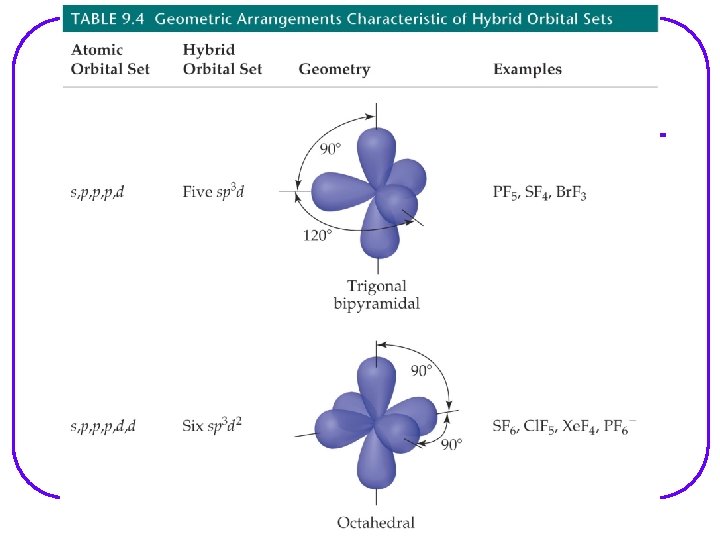
VSEPR Notation A = central atom l X = terminal atoms l E = lone pair of el AX 4 AX 3 E AX 2 E 2

We are going to go through all possibilities for: number of e- groups e- geometry molecular geometry VSEPR notation bond angles examples of each If gone when going over, some examples are as follows. However, VSEPR notation and bond angles are not shown.
![Determine the complete geometry and notation for: [ICl 4]-1 Determine the complete geometry and notation for: [ICl 4]-1](http://slidetodoc.com/presentation_image/34e482337f11a75ecbde135cb99fc122/image-11.jpg)
Determine the complete geometry and notation for: [ICl 4]-1
Determine the complete geometry and notation for: SO 2
When there is more than one central atom, consider the geometry and notation around each separately. Determine the complete geometry and notation for each central atom in: CH 3 NCO
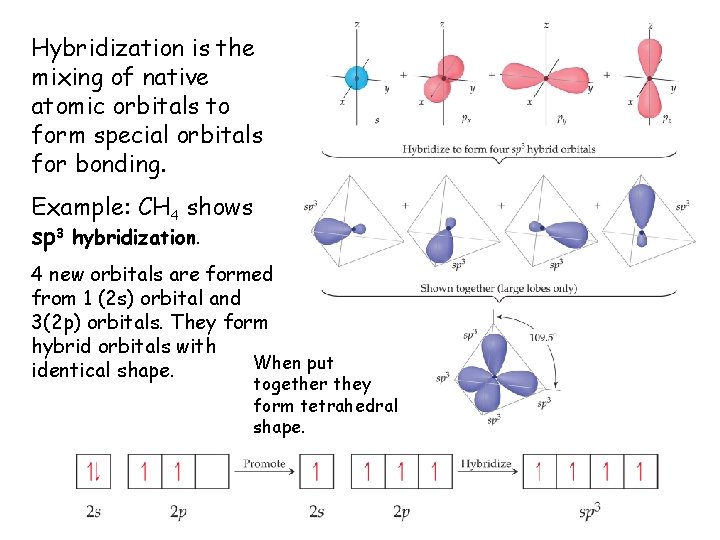
Hybridization is the mixing of native atomic orbitals to form special orbitals for bonding. Example: CH 4 shows sp 3 hybridization. 4 new orbitals are formed from 1 (2 s) orbital and 3(2 p) orbitals. They form hybrid orbitals with When put identical shape. together they form tetrahedral shape.
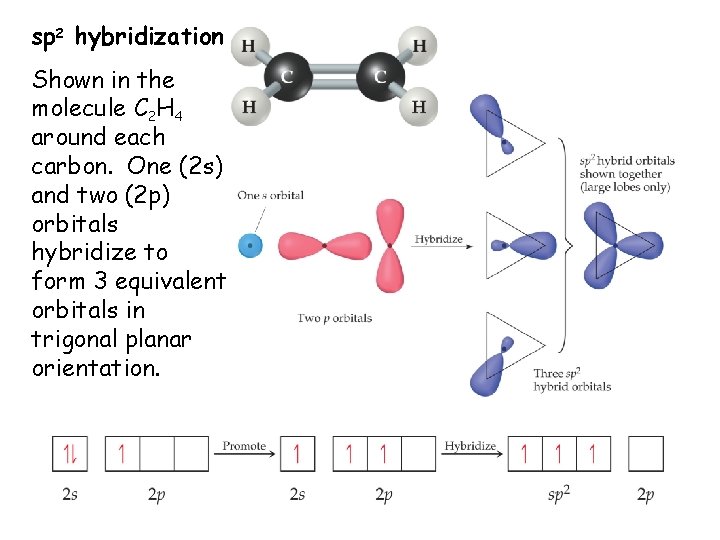
sp 2 hybridization Shown in the molecule C 2 H 4 around each carbon. One (2 s) and two (2 p) orbitals hybridize to form 3 equivalent orbitals in trigonal planar orientation.

sp hybridization shown in the carbon of CO 2, it has one (2 s) and one (2 p) orbital hybridized to form 2 equivalent orbitals. The molecule becomes linear. O=C=O
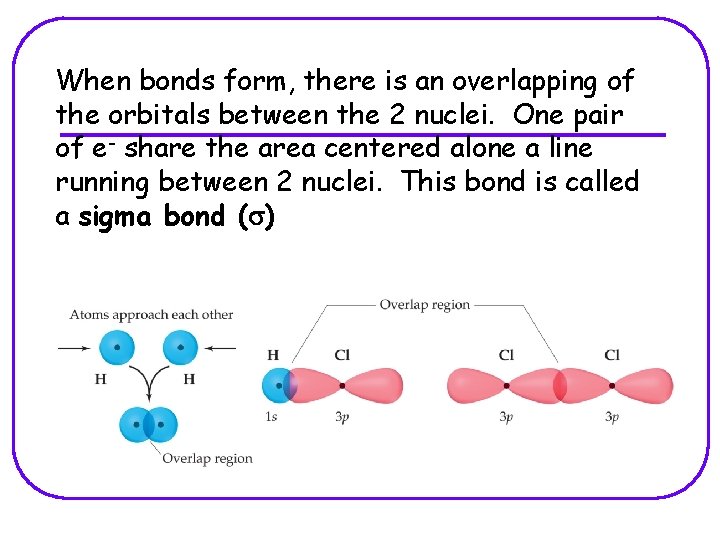
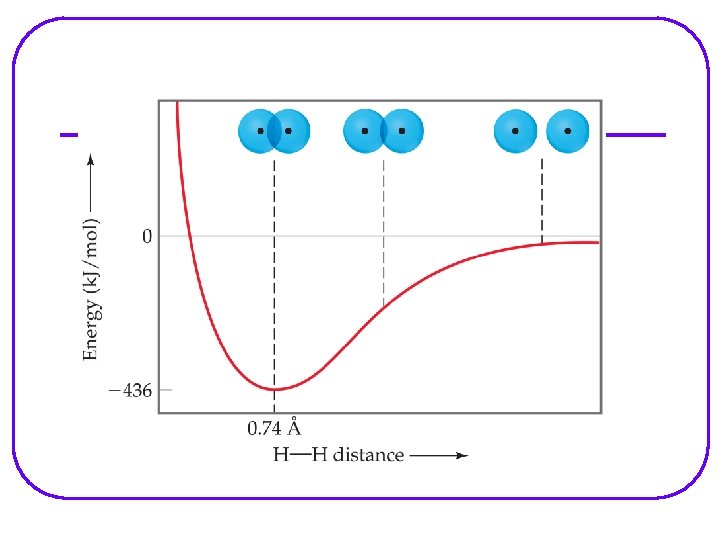
When bonds form, there is an overlapping of the orbitals between the 2 nuclei. One pair of e- share the area centered alone a line running between 2 nuclei. This bond is called a sigma bond (s)
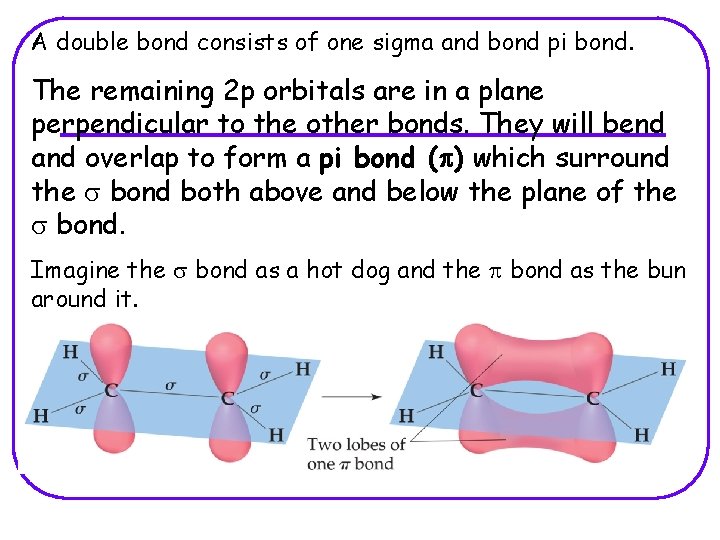
A double bond consists of one sigma and bond pi bond. The remaining 2 p orbitals are in a plane perpendicular to the other bonds. They will bend and overlap to form a pi bond (p) which surround the s bond both above and below the plane of the s bond. Imagine the s bond as a hot dog and the p bond as the bun around it.
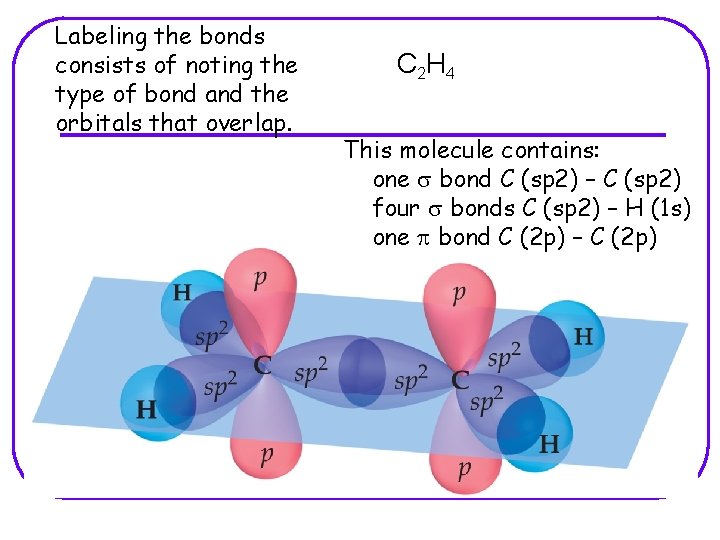
Labeling the bonds consists of noting the type of bond and the orbitals that overlap. C 2 H 4 This molecule contains: one s bond C (sp 2) – C (sp 2) four s bonds C (sp 2) – H (1 s) one p bond C (2 p) – C (2 p)
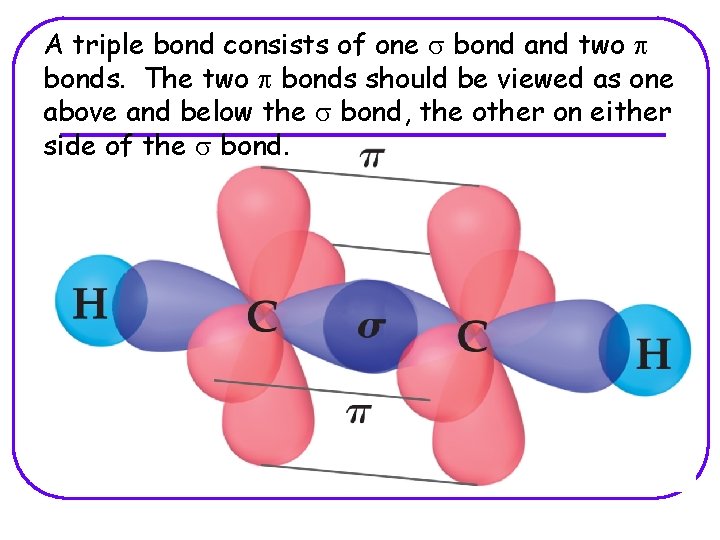
A triple bond consists of one s bond and two p bonds. The two p bonds should be viewed as one above and below the s bond, the other on either side of the s bond.
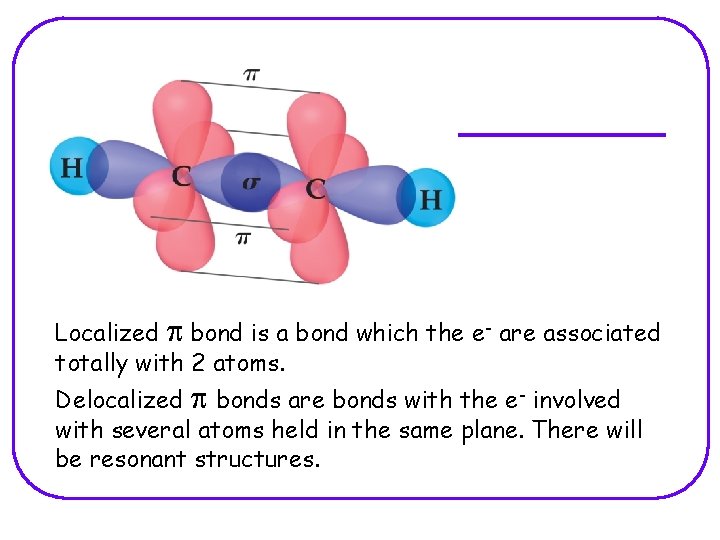
Localized p bond is a bond which the e- are associated totally with 2 atoms. Delocalized p bonds are bonds with the e- involved with several atoms held in the same plane. There will be resonant structures.

Molecular Orbitals (MO Theory) Overlap of 2 orbitals in a molecule create: 1. Constructive combination bonding MO Concentration of e- exist between two nuclei Lower energy More stable! 2. Destructive combination antibonding MO Concentration of e- exist on opposite sides of 2 nuclei Higher energy Bond order = ½ (# of bonding e- - # of antibonding e-)
Magnetic Properties l Diamagnetic elements have all e- in pairs, therefore all individual magnetic properties resulting from electrical spin is cancelled. Diamagnetic elements are repelled by magnetic fields so they will weigh slightly less in a magnetic field. l Paramagnetic elements have unpaired e-, therefore the individual magnetic properties resulting from electrical spin is not cancelled. There is an induced magnetic field due to the spin of the unpaired e-. Paramagnetic elements are attracted by a magnetic field so they will weigh slightly more in a magnetic field.
- Slides: 30